A Case Report of Acute Respiratory Distress Syndrome and Rhabdomyolysis in Covid-19 Disease: An Interchange of Causes and Effects
Author'(s): Loubelle B. Rirao MD1, Jeremy Owen G. Go MD1*, Ronald S. Perez, MD, MBA, FPCP, FPSN2 and Glynna Ong-Cabrera, MD, FPCP, FPCCP3
1Medical Resident, Department of Internal Medicine, Capitol Medical Center, Quezon City, Philippines.
2Nephrologist (Adviser), Department of Internal Medicine, Capitol Medical Center, Quezon City, Philippines.
3Pulmonogist (Adviser), Department of Internal Medicine, Capitol Medical Center, Quezon City, Philippines.
*Correspondence:
Jeremy Owen G. Go MD, Medical Resident, Department of Internal Medicine, Capitol Medical Center, Quezon City, Philippines, Tel: +63-9178618832 / +63-9275095276.
Received: 20 June 2021; Accepted: 28 July 2021
Citation: Rirao LB, Go JO, Perez RS, Ong-Cabrera G. A Case Report of Acute Respiratory Distress Syndrome and Rhabdomyolysis in COVID-19 Disease: An Interchange of Causes and Effects. Cardiol Vasc Res. 2021; 5(4): 1-4.
Abstract
Coronavirus Disease 2019 (COVID-19) is an emerging disease from SARS-CoV2 that can cause acute respiratory distress syndrome (ARDS) that can present with extrapulmonary symptoms such as rhabdomyolysis. This is a case of a 55-year-old male known case of pulmonary tuberculosis (PTB) recently started on fixed dose combination therapy admitted due to non-rotatory dizziness and diaphoresis. He had hyponatremia (119mmol/L) and was given Tolvaptan 15mg OD. In the interim, he was noted to have myalgia, weakness, fever and watery diarrhea. COVID-19 RT-PCR swab was positive. CK-MM (52.11U/L) and CPK-total (2954U/L) levels were elevated. He was managed as a case of rhabdomyolysis and PTB medications were withheld. Chest x-ray showed bilateral infiltrates. Inflammatory markers showed elevations in LDH 734U/L, CRP 62mg/L, Ferritin 4374.08ng/mL and Procalcitonin 0.12ng/mL. ABG showed respiratory alkalosis with severe hypoxemia (pO2 43mmHg). Patient was started on ceftriaxone 2gm IV OD, remdesivir (200mg IV loading dose, then 100mg IV OD), dexamethasone 6mg IV OD, hemodialysis and hemoperfusion, convalescent plasma (2 aliquots), enoxaparin 0.6cc SC OD, and was hooked to high flow nasal cannula (FiO2 80%, Flow 30LPM, Temp 35°C). During the course of admission, HE HAD atrial fibrillation in rapid ventricular response, hypomagnesemia (0.75mmol/L), hypokalemia (2.9 mmol/L), and acute liver injury (AST 142U/L, ALT 116U/L), and was managed with colchicine 0.5mg/tab OD, trimetazidine 35mg/tab BID, digoxin 0.25mg IV (q4 hours for 6 doses then OD), ivabradine 7.5mg/tab BID, magnesium and potassium supplementation, and ademetionine 300mg/tab 2 tablets TID. Patient’s symptoms resolved and was weaned from oxygen support, and underwent pulmonary rehabilitation (incentive spirometry, musculoskeletal training/exercises) then discharged. In this article, we discussed the correlation of ARDS and rhabdomyolysis to COVID-19 and its implications on patient’s course of illness and recovery.
Keywords
Introduction
Coronavirus Disease 2019 (COVID-19) is an emerging infectious disease crisis known to mainly affect the respiratory system. Alongside this, it can also present with several extrapulmonary manifestations such as diarrhea, myalgia, headache and abdominal pain. Since COVID-19 has a wide spectrum of presentation— asymptomatic, mild to severe to critical—patients with severe respiratory symptoms admitted in intensive care units develop long-term symptoms such as persistent cough, low-grade fever, breathlessness, fatigue, pain, myalgia [1], neurocognitive difficulties, and inability to return to normal activities [2]. It is not yet determined if there is a causal relationship with the development of rhabdomyolysis from COVID-19. In this case report, we describe a patient with critical COVID-19 presenting with rhabdomyolysis and acute respiratory distress syndrome managed with hydration and pulmonary rehabilitation in addition to the conventional treatment of COVID-19 such as O2 support, steroids and antiviral medications.
Case
This is a case of a 55-year-old male who came in with one-day history of non-rotatory dizziness and diaphoresis with no associated fever, myalgia, dyspnea, or chest pain. He was recently diagnosed with pulmonary tuberculosis and is currently on quadruple anti- Koch’s fixed dose combination therapy (Isoniazid /Rifampicin/ Ethambutol/Pyrazinamide) for 2 weeks. He is a 20-pack-year smoker and occasionally drinks alcoholic beverages. No other comorbidities were present. Persistence of dizziness prompted ER consult with vital signs of BP of 120/80, HR of 85, RR of 22, afebrile with oxygen saturation of 98% at room air. His physical and neurologic examination were unremarkable.
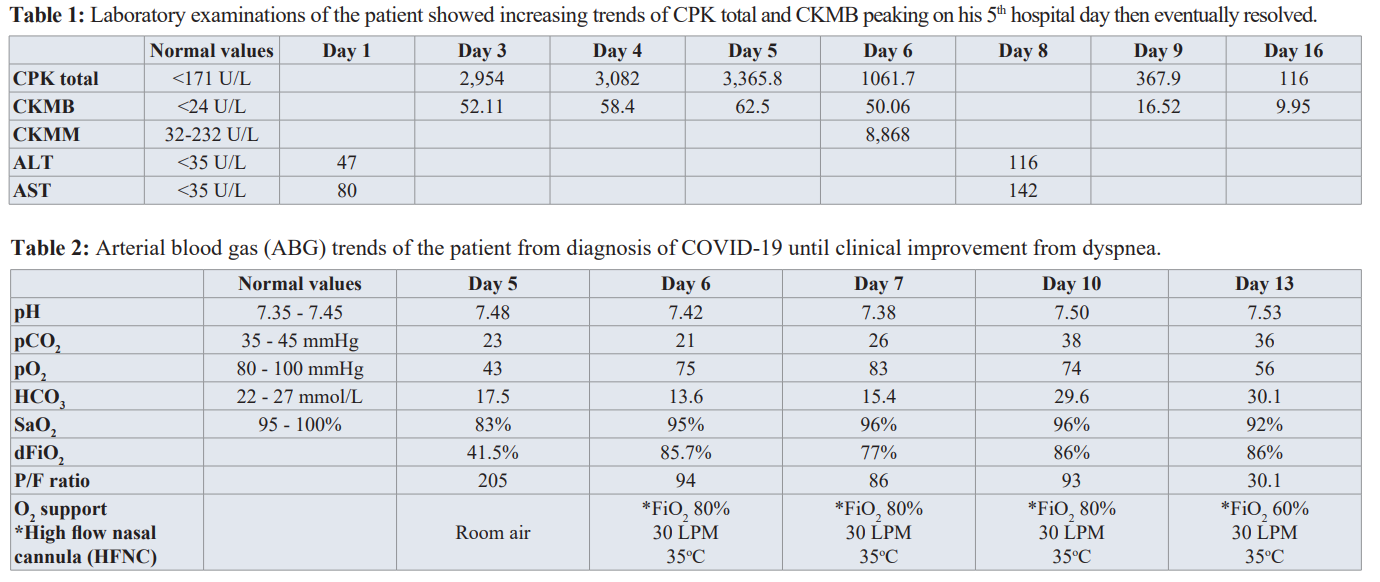
Initial workup showed his ECG having normal sinus rhythm and his chest radiograph showed reticular opacities in the left upper lung due to pulmonary scarring. His CBC, urinalysis, and serum potassium were normal. His creatinine was 93 μmol/L with eGFR of 80 mL/min/1.73m2. His serum sodium is low at 119 mmol/L with urine sodium of 31.2 mEqs/L. He was started on Tolvaptan 15mg/tab once daily with serial monitoring of serum sodium. During his hospital stay, the patient experienced fever, loose watery stools, mild abdominal pain and myalgia. The patient persistently complained of muscle pain and generalized weakness. Hence, CK -MB and CPK-Total were requested and the results were significantly elevated. Serial CK-MB and CPK-total showed increasing trends as shown in Table 1. Rhabdomyolysis was considered and HRZE was discontinued. On his 3rd hospital day, patient continued to have watery stools with fever and myalgia. A COVID RT-PCR test was done which showed a positive result. On the 4th hospital day, patient was observed to be weak looking with some disorientation. The patient had episodes of desaturation down to 83% but still with vesicular breath sounds. Repeat chest radiograph showed and the presence of pneumonic infiltrates in the right lower lung (Figure 1 left).
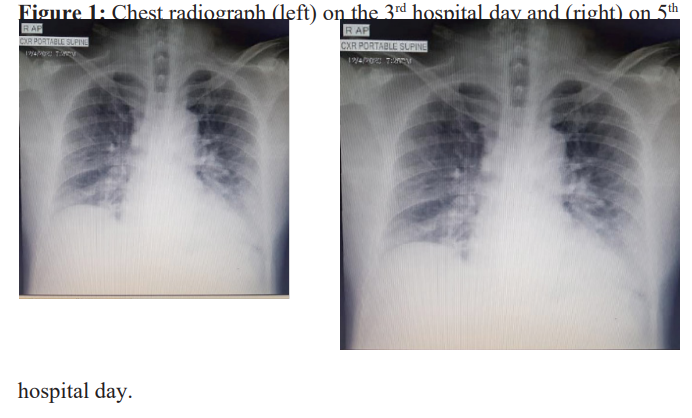
Arterial Blood Gas done showed Mixed respiratory and metabolic alkalosis with severe hypoxemia (see Table 2, Day 13). Chest X-ray showed progressive increase in bilateral infiltrates (Figure 1, right). Inflammatory markers were mostly elevated: LDH 734 U/L, CRP 62 mg/L, Ferritin 4374.08 ng/mL and Procalcitonin 0.12 ng/mL. The patient was then transferred to intensive care unit and was initially started on ceftriaxone 2g IV OD and was started on High Flow Nasal Cannula with an initial setting of Flow rate of 30 lpm, and Fio2 60 % and Dexamethasone 6mg IV OD was started. There was persistence of desaturation, myalgia, and loose watery stools. Serial monitoring of CK-MB and CPK total were continued. CKMM and LDH were elevated as seen in Table 1. Remdesivir 200mg IV loading dose then 100mg IV once daily and Azithromycin 500mg IV once daily were given. Prone positioning was also done.
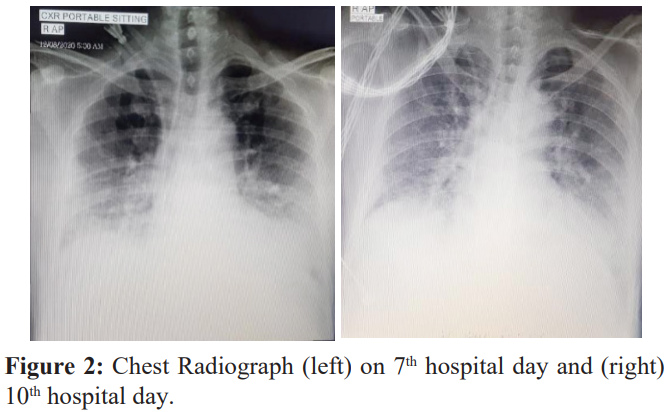
A repeat Chest X-ray was done on the 7th hospital day which showed progressive increase in infiltrates (Figure 2 left). Persistence of desaturation prompted hemodialysis with hemoperfusion (HD+HP). On his first HD+HP, the patient had episodes of hypotension and atrial fibrillation with rapid ventricular response. Troponin I showed elevation at 41.80 ng/L Serum K was low at 2.9 mmol/L and magnesium at 0.72 mmol/L. Enoxaparin 0.6cc SC once a day, colchicine 0.5 mg once a day, trimetazidine 35mg 1 tab twice daily, digoxin 0.25mg once daily and ivabradine 7.5 mg twice daily were started. Amiodarone drip initially started but was shifted to magnesium drip. Potassium and magnesium were corrected via supplementation (oral/drip). Liver enzymes were noted to be elevated: AST 142 U/L, ALT 116 U/L; hence, patient was started on Ademetionine 300mg/tab 2 tablets 3 times a day. Oxygen support using high-flow nasal cannula was increased to Fio2 80% with a flow rate of 30 L/min. On the 8th hospital day, the patient was still weak-looking and with episodes of desaturation and tachycardia. He was then given COVID convalescent plasma (2 aliquots of 250cc each bag). Ceftriaxone was shifted to piperacillin-tazobactam 4.5mg every 6 hours. On his 10th hospital day, chest Xray showed interval clearing of bilateral infiltrates (Figure 2, right). Present antibiotics and Oxygen supplementation were continued. Pulmonary rehabilitation was started which included incentive spirometry with instruction to do pursed lip breathing and diaphragmatic breathing. Active assisted range of motion exercises were also done. Patient underwent Hemodialysis with Hemoperfusion for 4 consecutive days.
On the 14th hospital day, the patient’s oxygenation improved along with his myalgia. The patient was gradually weaned from the HFNC and shifted to nasal cannula at 2lpm. He was then transferred back to ward. On his 16th hospital day, CK-MB and CPK total decreased to normal range (Table 1). Incentive spirometry and exercise training involving the upper and lower exercises were progressed. With the improvement of his clinical status, was eventually discharged on his 25th hospital day.
Discussion
COVID-19 infection mostly affects the respiratory system; however, some extrapulmonary symptoms were also documented such as myalgia [3] which was present in our patient as a result of Rhabdomyolysis. The patient did not have any history of any strenuous activity prior to disease onset, alongside trauma, alcohol or illicit drug use. CK levels starts to rise within 12 hours of the onset of muscle injury and peak at 24-72 hours and return to the baseline over three to five days [4]. In our patient, his myalgia started to manifest from day 2 of illness with CK total levels of 2,954 IU/L, peaking on day 5 of illness at 3,365 IU/L, and then eventually had a downward trend afterwards including resolution of his myalgia.
Rhabdomyolysis is rarely seen and was only reported in 0.2% of patients in a study of 1099 patients in China [5]. Although rare, rhabdomyolysis is a potentially life-threatening complication because it can cause renal failure, hyperkalemia and metabolic acidosis. Aggressive intravenous fluid hydration, correcting electrolytes, monitoring urine output, serial muscle enzymes, and urine pH monitoring are required in the treatment of rhabdomyolysis [4]. Some patients may require dialysis to correct acidosis or hyperkalemia, as what the patient underwent with Rhabdomyolysis is one of the few complications of COVID 19 [6]. It is a breakdown of the myocytes of skeletal muscles and the release of intracellular contents characterized by increased levels of CK, myoglobin, potassium, aldolase, lactate dehydrogenase, urate, and ALT [7]. Several types of musculoskeletal cells express the ACE2 and TMPRSS2 genes which allow for direct viral invasion and infection [6,8] and several of the proinflammatory signaling molecules known to be elevated in patients with COVID-19 can also impact skeletal muscles such as IFN-g, IL-1b, IL-6, IL-17, and TNF-a which can directly induce muscle fiber proteolysis and decrease protein synthesis leading to rhabdomyolysis [6,8- 9]. Other mechanism may include exaggerated immunological reaction from cytokine storm, and T-cell-guided myocyte damage [6]. In diagnosing rhabdomyolysis, laboratory evaluation supplements the clinical history (other causes of rhabdomyolysis must be excluded such as trauma, use of illicit drugs, alcohol, seizures, etc.).
The Patient was recently diagnosed with pulmonary tuberculosis and was currently on quadruple anti-Koch’s (Isoniazid / Rifampicin / Ethambutol/ Pyrazinamide). It is known that anti- TB medications particularly pyrazinamide can cause significant myalgia as its side effect [10-11]. There were a few case reports that has cited pyrazinamide to have caused rhabdomyolysis. The first case rhabdomyolysis associated with pyrazinamide was first reported in 1991 [11]. The patient having both conditions, PTB and COVID-19 infection may have led to the development of rhabdomyolysis.
Since COVID-19 patients experience immobilization due to prolonged hospitalization and bedrest in addition to physical inactivity due to sustained quarantine and social distancing, there is increased risk of damage to the human body’s systems, in particular the musculoskeletal system, that can impact the severity of COVID-19 [12]. These patients are at high risk of several adverse health effects from decreased respiratory movement and muscle weakness after prolonged hospitalization [5]. Most patients post ICU care have difficulty recovering their pre-morbid strength and pulmonary functional capacity impairing their quality of life since ARDS is one of the most common complications of severe COVID-19 infection [13]. Restrictive pulmonary problems may develop due to pulmonary fibrosis and weakness of the respiratory muscles. Long-term effects of ARDS are evaluated with spirometry, diffusion capacity, and cardiopulmonary functional capacity [13]. Some of the goals of pulmonary rehabilitation in patients post-ARDS is to maintain the respiratory flow, decrease dyspnea, improve endurance and general exercise tolerance and to recover functional loss to improve quality of life [13]. Pulmonary rehabilitation exercises include respiratory muscle training and controlled breathing techniques such as incentive spirometry and other aerobic/endurance exercises. In our patient’s case, he was started on incentive spirometry and was referred to rehabilitation service for skeletal muscle training and exercises [13].
Our patient experienced generalized muscle weakness, significant myalgia, and easy fatigability and are all-secondary to COVID-19 infection. Additional to these are the history of taking anti- Koch’s medication and prolonged hospital stay and immobilization/ inactivity leading to decreased muscle strength and muscle atrophy. Aside from treating patients with antiviral medications, steroids, oxygen supplementation and hemoperfusion, it is also important to provide pulmonary rehabilitation exercises to improve their quality of life.
Conclusion
While pulmonary complications are well-documented in patients contracting COVID-19, other extrapulmonary manifestations such as rhabdomyolysis may arise. Standard treatment included antiviral medications, steroids, oxygen supplementation and hemoperfusion, but it is also important to provide pulmonary rehabilitation exercises to improve their quality of life.
Footnote
The case report was shown as poster presentation at the Philippine College of Chest Physicians 40th Annual Chest Convention Virtual Conference held last March 2-5, 2021.
References
- National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2020.
- Chopra V, Flanders SA, O’Malley M, et al. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2020.
- Sreenath Meegada, Vijayadershan Muppidi, Donald C Wilkinson, et al. Coronavirus-induced Rhabdomyolysis. 2020.
- Sime PJ, O’Reilly KM. Fibrosis of the lung and other tissues: New concepts in pathogenesis and treatment. Clin. Immunol. (Orlandofla) 2001; 99: 308-319.
- Guan Wj, Ni ZY, Hu Y. Liang WH, et al, Clinical Characteristics of coronavirus disease 2019 in China. N Eng Med 2020.Meegada S, Muppidi V, Wilkinson DC, et al. Coronavirus Disease 2019-Induced Rhabdomyolysis. Cureus. 2020; 12:10123.
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019; 17: 181-192.
- Markus Hoffmann, Hannah Kleine-Weber, Simon Schroeder, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181: 271-280.
- Reid MB, Li YP. Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res. 2001; 2: 269-272.
- Kim JH, Wallerstein S, Thoe M, et al. Myopathy in Tuberculosis: Two Presumptive Cases and a Review of the Literature. Military medicine. 1997; 162: 221-224.
- Namba N. A Case of pyrazinamide -associated myoglobinuria renal failure. Jpn J Med. 1991.
- Jeffrey A.Woods, Noah T.Hutchinson, Scott K. Powers, et al. The COVID-19 Pandemic and physical activity. Sports Medicine and Health Science. 2020; 2: 55-64.
- Pulmonary rehabilitation principles in SARS-COV-2 infection (COVID-19): A guideline for the acute and subacute rehabilitation. 2020.