A Dose Response Analysis of Dual Renin Angiotensin Aldosterone System (RAAS) Blockade Among Diabetic Nephropathy Patients with Albuminuria and Proteinuria: A Meta-Analysis
Author'(s): Sultan Al Dalbhi MD1*, Mohammad Al Eissa MD2 , Khuzama Al Kayid MD2, Malak Alkhodaidi MD3, Malak Albalawi MD3 and Ghadeer Althaqib3
1Department of Adult Nephrology, Prince Sultan Military Medical City, Riyadh 11159, Saudi Arabia.
2Department of Family Medicine, Prince Sultan Military Medical City, Riyadh 11159, Saudi Arabia.
3College of Medicine, Princess Nourah Bint Abdulrahman University, Riyadh 84428, Saudi Arabia.
*Correspondence:
Sultan Al Dalbhi, Prince Sultan Military Medical City, Riyadh 11159, Saudi Arabia, Tel: +966 53037 3337; Fax: +966 114777714.
Received: 15 February 2019; Accepted: 04 March 2019
Citation: Sultan Al Dalbhi, Mohammad Al Eissa, Khuzama Al Kayid, et al. A Dose Response Analysis of Dual Renin Angiotensin Aldosterone System (RAAS) Blockade Among Diabetic Nephropathy Patients with Albuminuria and Proteinuria: A Meta-Analysis. Diabetes Complications. 2019; 3(1): 1-11.
Abstract
Objective: Treatment with renin angiotensin aldosterone system (RAAS) blockade including angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs) have been shown to improve clinical outcomes. However, recent contrasting evidence regarding the dual RAAS blockade has also been presented. Very few studies have investigated the effectiveness of this dual blockade among diabetic nephropathy (DN) patients in association with albuminuria or proteinuria.
Research Design and Methods: A review of randomized controlled trial (RCT) studies (n=45) reporting on the dose response analysis among DN patients using the RAAS blockade (including both ACEi and ARBs and other combinations) and other monotherapies over a 25-year period was performed. Overall, 45 studies of DN patients (n=18,628) with albuminuria or proteinuria were included.
Results: An association between dual RAAS blockade and DN was observed, in which 18 of the 45 datasets revealed that combination therapies were effective among DN patients. Although there was a decline in albuminuria (mean difference: -19.93 mcg/L; 95% CI -50.32 – 10.47; I2 = 87.8%, p = 0.000) and a slight decline in proteinuria (mean difference: -0.19 mg/mmol; 95% CI -2.32 – 2.70; I2 = 99.2%, p = 0.000) with dual RAAS blockade combination therapy, these results demonstrated high heterogeneity among studies, with non-significant effects.
Conclusion: Based on this study, it appears that dual RAAS blockade (or a combination of therapies) is a neutral treatment for patients with DN presenting with symptoms of albuminuria and/or proteinuria. Therefore, other factors must be considered when recommending therapies for DN patients.
Keywords
Background
Type 2 diabetes mellitus is a debilitating disease and the leading cause of end-stage renal disease worldwide [1]. Earlier evidence suggests that treatment with renin angiotensin aldosterone system (RAAS) inhibitors, including angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs), have been shown to improve clinical outcomes among diabetic patients with hypertension, while also reducing the incidence of microalbuminuria in patients with normoalbuminuria [2,3], the progression to overt proteinuria in patients with microalbuminuria [4-6], and the development of end-stage renal disease in patients with overt nephropathy [1].
A systematic review study published in 2012 showed that dual RAAS inhibition is an option to decrease proteinuria and control BP in patients with diabetic kidney disease (DKD) but is associated with an increased risk of hyperkalemia [7]. In their meta-analysis, it was demonstrated that an increase in serum potassium was less pronounced with combined ACEi/ARB than with other combinations. At the time, it was suggested that therapy should only be attempted with ACEi/ARB combination and only in selected patients (i.e. those with macroalbuminuria and normal serum potassium levels on RAAS blockade monotherapy) [7].
Contrasting evidence on the dual RAAS blockade
The latest research demonstrates that the long-held belief that a more complete blockade of RAAS, with a combination of two of the three existing RAAS blockers (ACE inhibitors (ACEi), ARBs, or DRIs), has come under serious doubt regarding its effectiveness and safety for the treatment of patients with hypertension, or nephropathy with proteinuria [8]. With regards to clinical studies, it was concluded that, in human diseases, there are currently no proven benefits of the combined ACEi and ARB over single drug RAAS blockade. Given the high-risk of serious complications, it was suggested that dual RAAS blockade cannot be currently recommended as the therapy of choice, even in patients with heavy proteinuria. This has also been confirmed in large medical trials and meta analyses, which is considered to be evidence based [9]. Fried et al. also demonstrated that the use of combination therapy with an ACE inhibitor and an ARB among patients with proteinuric DKD does not provide an overall clinical benefit.
In addition, available data suggests that a dual blockade of RAAS is not currently feasible among diabetic patients with diabetic nephropathy (DN). This does not mean that in future, a dual blockade (ACEi plus ARBs) should not be used in these patients, but that this therapeutic approach should be tested among selected diabetic populations to identify subgroups of patients with whom the desired nephro- and cardio-protection are achieved without increase in side effects (10). The results of these previous studies revealed that ONTARGET, ALTITUDE, and VA NEPHRON-D do not allow the formulation of definitive considerations on the role of a dual blockade of RAAS with ACEi and ARBs among diabetic patients with microalbuminuria or proteinuria. This is because they were heterogeneous studies with short follow-up durations and weak end-points [10]. Although a study conducted by Elrggal et al. was selective and somewhat biased, the authors make compelling arguments that cast serious doubt over the strength of the evidence upon which the current guidelines are based, favoring the use of dual RAAS blockade among DKD patients [11].
Regarding albuminuria, a few studies suggest that albuminuria should not be considered as a target for treatment, but instead a surrogate marker of DKD progression, as it is unknown whether the adverse effects of combination therapy will offset any benefit [11,12]. It was also suggested that reduced albuminuria does not always translate to a decrease in cardiovascular and renal morbidity. However, dual therapy carries an increased risk [10].
Over the past five years, interventions with a dual RAAS blockade may have improved or shown positive or negative effects among diabetic patients. As such, there is little evidence that evaluates the dual RAAS blockade therapies among DN patients with albuminuria or proteinuria. Therefore, this review attempts to fill this research void by providing a transparent overview of dual RAAS blockade and monotherapies in modern day medicine.
Research Design and Methods
A systematic review and meta-analysis were conducted according to Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guidelines [13].
Data Sources and Searches
A review of randomized controlled trials (RCT) studies (n = 45) reporting on the dose response analysis among DN patients using dual RAAS inhibitors including both ACEi and ARBs or other monotherapies over a 25-year period (1993 – 2017) was performed. We felt that it was imperative to also include RCTs using monotherapy, in order to provide a transparent overview of the therapeutic effect among DN patients. The following databases were searched: NHS evidence, EMBASE, Medline and PubMed, Google Scholar, and the Cochrane Library. The search items were based on established terminology using Cochrane definitions where possible and were “diabetic nephropathy” and “renin angiotensin aldosterone system;” “RAS/RAAS blockade” and “albuminuria;” “RAS/RAAS blockade” and “proteinuria.”
The titles and/or abstracts were reviewed to exclude any clearly irrelevant studies. The full texts of the remaining studies were then retrieved and read in full, independently, to determine whether the studies met inclusion criteria. The reference lists of studies that examine the topic of interest were checked for additional publications.
Criteria for inclusion in the review
Abstracts were considered eligible for full manuscript data extraction if the study met all the following criteria: a) they reported an association or dissociation of a dose response analysis between either dual RAAS blockade or monotherapy and DN; b) the sample consisted of adults (>18 years of age); and c) the study design was cross-sectional or randomized controlled trials (RCTs). Studies that solely consisted of patients with type 1 or 2 diabetes (without nephropathy, albuminuria, or proteinuria) were not included. Our inclusion criteria revealed 45 studies of DN patients with either albuminuria or proteinuria.
Data extraction
Using a standardized data extraction sheet, the following information (if available) was extracted and recorded from the studies: authors; year of publication; country of origin; study design; total sample size of participants; methods of assessment/ experiment; outcomes; effective treatments. If multiple risk estimates were presented in a given manuscript, the unadjusted estimate was selected for the primary meta-analysis as some studies were adjusted for prominent confounding variables, while others were not, rendering a direct comparison of estimates to be questionable.
Quality assessment
The PRISMA guidelines for RCTs (13) were used to examine the quality of the studies. These include adequacy of study design (RCT with an adequate control group); recruitment of sample; ascertainment of diabetes, albuminuria and RAAS inhibitors; and control for co-founding variables, such as BP, and estimated glomerular filtration rates (eGFR). The quality of the studies was not summarized with a score, as this approach has been criticized for allocating equal weight to different aspects of methodology [14], but a formal assessment of the risk of bias and strength of evidence according to the Agency for Healthcare Research and Quality (AHRQ) guidelines was conducted [15]. A study was considered to be of high quality if the study design was prospective in nature; consecutive or a random sampling method was used; and cofounders for DN, albuminuria and proteinuria, systolic BP (SBP), diastolic BP (DBP), and eGFR were accounted for.
Data Synthesis and Analysis
The primary outcome analyzed was the mean difference in percent reduction in proteinuria and albuminuria between the combination therapy and monotherapy groups. In some studies, proteinuria and albuminuria outcome data were presented as the geometric mean and 95% confidence interval (CI). In studies which had two monotherapy comparator arms (eg. ACEi or ARB, enalapril or losartan, telmisartan or valsartan, spironolactone or control), the average of the means and standard errors of the two arms was utilized. Secondary outcomes included changes in SBP, DBP, and GFR.
We fitted a random effects model to the study data as it includes estimates taken from a series of independently performed studies. We interpreted I2 results as low, moderate, and high heterogeneity represented by 0-25%, 26-50%, and >50%, respectively. Forest plots were created with STATA 14.2 (StataCorp (College Station, Texas USA) and are reported with standard errors (SE) and 95% confidence intervals for mean differences.
Results
Study selection
The flowchart for study inclusion is shown in Figure 1. The literature search resulted in 987 studies. After review of their titles and abstracts, 152 studies met the initial inclusion criteria and were retrieved for full text review. Of these, 107 studies were excluded from the systematic review as they no longer met the inclusion criteria. A total of 45 studies were included in the systematic review, and the extracted data are summarized in Table 1. The risk of bias of the studies (n = 18) was included in the meta-analysis using the Cochrane assessment tool (Table 2).
Qualitative summary
A standard data extraction template was created using Microsoft
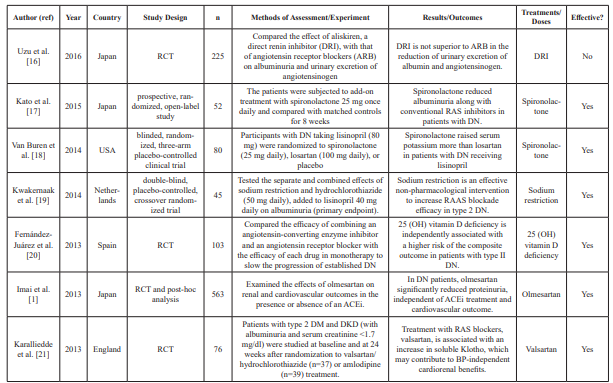
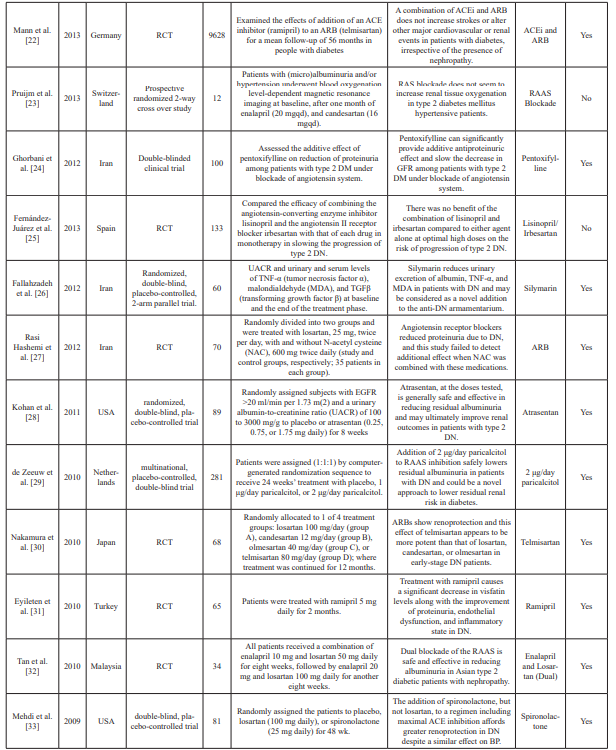
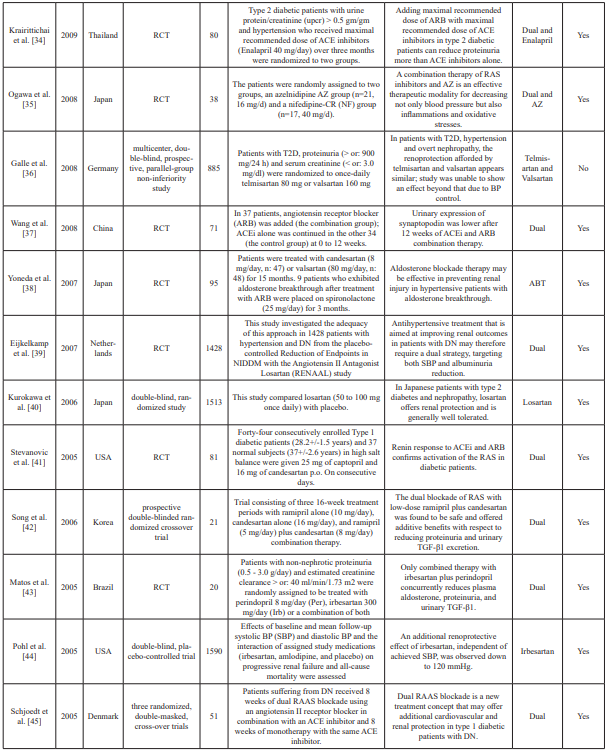
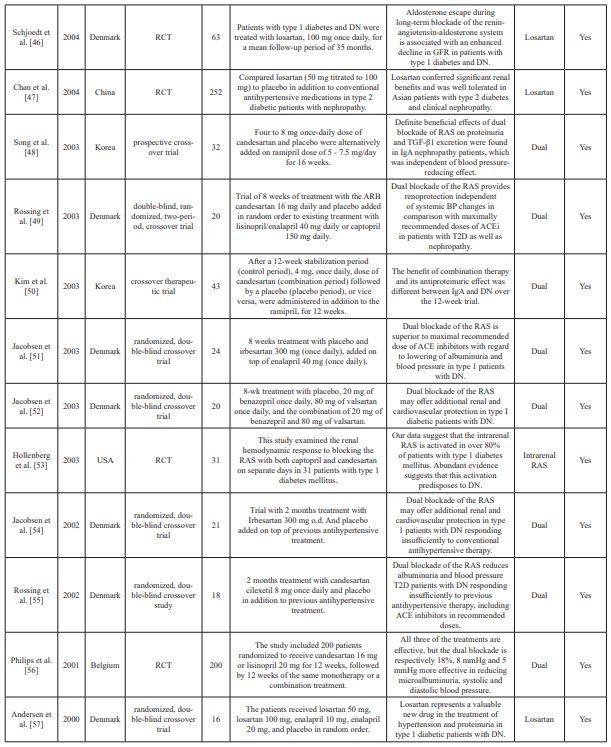

Table 1: Summary table of studies included in the systemic review.
n: Sample size; PRA: Plasma renin activity; Aldo: Aldosterone; AZ: azelnidipine; ACEi: Angiotensin-converting enzyme inhibitors; ABT: Aldosterone blockade therapy; ARB: Angiotensin II receptor blocker(s); RCT: Randomized clinical trial(s); DRI: Direct renin inhibitor; RAS: Renin-angiotensin; RAAS: Renin-angiotensin aldosterone system; mg: milligrams; OH: Hydroxy; NIDDM: Non-insulin dependent diabetes mellitus; T2D: Type 2 diabetes; DM: Diabetes mellitus; DN: Diabetic nephropathy; μg: Microgram; UACR: Urine to albumin creatinine ratio; MDA: Methylenedioxyamphetamine; TGF: Transforming growth factor; TNF: Tumor necrosis factor; DKD: Diabetic kidney disease; BP: Blood pressure; GRF: Glomerular filtration rate; eGFR: Estimated glomerular filtration rate; ref: Reference.
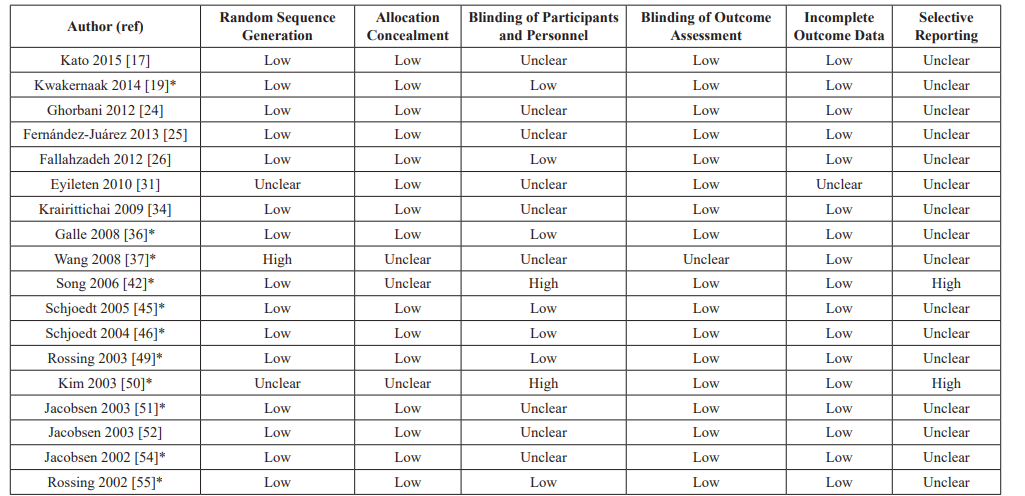
Table 2: Risk of bias in included studies for the meta-analysis based on the Cochrane Assessment Tool.
*Included in analysis for primary outcomes.
Excel. Data extraction included abstractions based on predefined categories as well as qualitative text data (to allow a combination of systematic assessment and depth to be achieved). The collected information included details about the study characteristics, such as aims, participants, study design, methods, outcomes, and treatment/dose measures. Two reviewers carried out data extraction independently and resolved any disagreement by consensus and discussion. Of the 45 datasets included in the meta- analysis, two were crossover study trials and five were prospective studies. Fifteen conducted double-blinded trials, and 23 conducted randomized trials. Data characteristics from the datasets are summarized in Table 1.
Risk of bias and strength of evidence
Given that most studies were RCTs, the overall risk of bias was moderate to high and the study quality was fair. The overall magnitude of association was high and there was large heterogeneity between studies. A possible reason for high heterogeneity (I2: 85%>) is that no correlations were made with regards to sub- groups or geographical regions in the included studies. The studies included for analysis were conducted in a number of countries (China, Denmark, Germany, Iran, Japan, Korea, Netherlands, Spain, Turkey, and Thailand).
Main findings
To our knowledge, this study presents one of the first systematic reviews and meta-analyses of the evidence for a neutral effect between dual blockade RAAS and DN using data from RCTs and controlled trials. An association (which was non-significant, due to the high heterogeneity) between a dual RAAS blockade and DN was observed; 18 of the 45 datasets revealed that dual therapies were effective among DN patients. The results appear to demonstrate high I2 values with significant associated p-values as well as CI that cross 0. This demonstrates high heterogeneity among the studies and a non-significant effect.
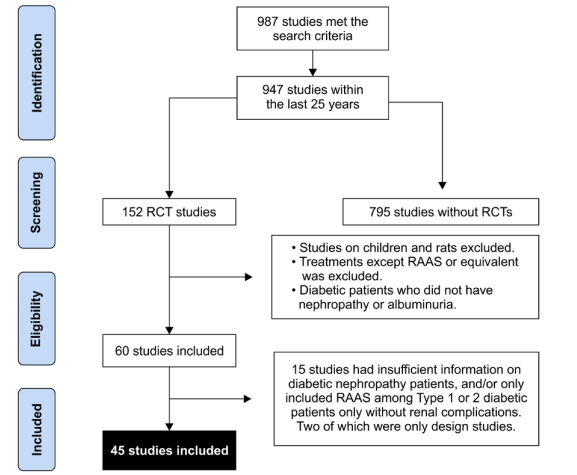
Figure 1: Preferred reporting items for systematic reviews and meta- analyses (PRISMA) flow diagram. Study identification and selection process.

Figures 2A: Decline in albuminuria with dual RAAS blockade combination therapy.
2B) Slight decline in proteinuria with dual RAAS blockade combination therapy.
2C) Slight decrease in GFR with dual RAAS blockade combination therapy.
The effect of combination therapy on albuminuria
There was a decline in albuminuria with dual blockade combination therapy (mean difference: -19.93 mcg/L; 95% CI -50.32 – 10.47; I2 = 87.8%, p = 0.000) (Figure 2A).
The effect of combination therapy on proteinuria
There was a slight but non-significant increase in proteinuria with dual blockade combination therapy (mean difference: 0.19 mg/mmol; 95% CI -2.32 – 2.70; I2 = 99.2%, p = 0.000) (Figure 2B). There was also a slightly but not significant decrease in GFR (mean difference: -1.11 mL/min; 95% CI -3.00 – 0.77; I2 = 95.0%, p = 0.000) with dual blockade combination therapy (Figure 2C).
The effect of combination therapy on blood pressure
There was a 1.33 mmHg decrease (mean difference: -1.33, 95% CI -3.70 – 1.04; I2 = 95.3%, p = 0.000) in SBP with dual blockade combination therapy (Figure 2D), and 1.58 mmHg decrease in DBP (mean difference: -1.58, 95% CI -3.25 – 0.14; I2 = 95.0%, p = 0.000) with dual blockade combination therapy (Figure 2E).
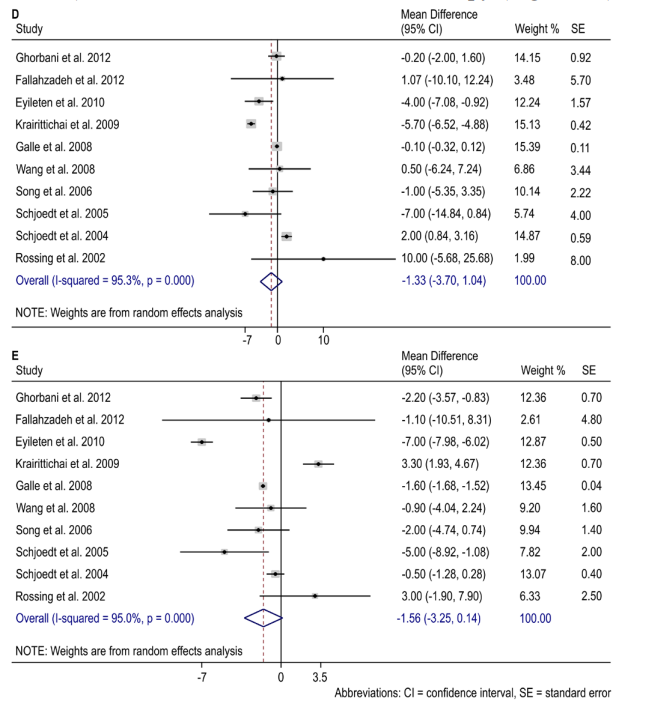
Figures 2D): Percent reduction in SBP with dual RAAS blockade combination therapy.
2E): Percent reduction in DBP with dual RAAS blockade combination therapy.
Abbreviations: CI = confidence interval, SE = standard error.
Discussion
Based on this review, it is reasonable to postulate that a further reduction in proteinuria, albuminuria, and BP by dual RAAS blockade (or combination therapies) might further decrease disease progression. In this meta-analysis, we found that the effects of combination dual RAAS blockade therapy were neutral for proteinuria and albuminuria among DN patients.
However, dual RAAS blockade was associated with a slight decline in SBP and DBP. This finding is consistent with a review conducted by Elrggal et al. on a number of key clinical trials, which highlighted the dogma that the benefit of a RAAS blockade in DKD are beyond mere adequate blood pressure control.
A number of studies (n = 18), studies combining ACEi and ARB particularly, demonstrate that dual blockade of the RAAS is safe and effective in reducing albuminuria, proteinuria, blood pressure, and urinary markers [22,32,34,35,37,39,41-43,45,48,49-52,54-56], as well as provide renal and cardiovascular protection [54]. These studies also suggest that dual blockade of the RAAS is more effective than individual treatments (monotherapies).
Four studies have specifically shown that the combination of ACEi and ARB provides renal and cardiovascular benefits in patients with DN [22,34,37,41]. Both ACEis and ARBs suppress aldosterone secretion; however, with prolonged treatment, aldosterone levels increase, a phenomenon termed “aldosterone escape” [7,46]. Moreover, there is a secondary increase in renin with either ACEi or ARB therapy. Interestingly, Pohl et al. also suggested an SBP target between 120 and 130 mmHg, in conjunction with blockade of the RAS, in patients with DN [44].
In contrast, there are also a number of studies (n = 21) which investigated the effect of using monotherapies, and report some benefits and effects on renal outcomes and/or RAAS blockade efficacy among DN patients [16-19,21,24,26- 31,33,38,40,46,47,53,57-59].
However, these studies do not report significant benefits of these therapies when used in combination. ARBs have also shown to be a more superior and potent treatment option compared to others among DN patients [16,30]. In six studies, the therapies appeared to have a similar effect or were unable to show any effect or benefit on varied renal outcomes [1,20,23,25,36,44].
Based on the current evidence, many investigators no longer recommend the routine use of dual RAAS blockade for the treatment of hypertension or chronic kidney disease, except for the presence of HF with reduced EF or DN with proteinuria [8]. Instead of implementing new methods non-selectively, the effort should be directed towards finding specific populations (defined by genetic markers, features of the disease, or other parameters) that will have the most benefit and/or the least risk from any particular treatment regime. The more distant future will probably bring the “tailoring” of the treatment to each individual, and not simply to a group or a disease [9].
Strengths and Limitations
The primary strength of this meta-analysis is the expansive literature search. However, there are several limitations, mainly stemming from the quality of the included studies, as summarized in Table 1 A few studies in the review contained a secondary analysis of a study designed to test a different primary hypothesis, and this will inevitably result in some measurement bias and residual confounding. The absence of patient outcome data necessitated the use of surrogate markers (proteinuria and albuminuria) for the primary outcomes. Further research should be conducted on correlating these outcomes to sub-groups or geographical regions. Furthermore, there was likely an underrepresentation of African patients being studied in the included studies. The strength of systematic reviews is that by systematically identifying these limitations, future designs can be improved.
Implications
This review suggests that we should evolve from repeating RCT studies to studies examining causal relationships. The ideal study design could either be a prospective design including patients potentially at high risk for type 2 diabetes mellitus (e.g., positive family history of diabetes); with or without DN, albuminuria or proteinuria; matched for at least positive changes in BP and GFR measured over time. Further secondary analyses are unlikely to contribute further to the field unless there is an adequate assessment of potential confounding factors.
Conclusion
This review contributes to the growing evidence of a moderate but persistent dose-response relationship for the efficacy of RAAS blockade among patients with DN. Based on this study, it appears that dual blockade RAAS (or a combination of therapies) is a neutral treatment for patients with DN presenting with symptoms of albuminuria and/or proteinuria, as other factors must also be considered when recommending therapies for DN patients.
Acknowledgments
We would like to thank Ms. Jenna Patterson for her assistance with the statistics, as well as Ms. Taahira Goolam Hoosen for internally reviewing the paper. Sultan Al Dalbhi is the guarantor of the manuscript.
Author Contributions
S.A. conceived and designed the study, obtained the data, proposed and performed the statistical analyses, contributed to the literature search, drafted the report, M.E reviewed/edited the manuscript, and revised the report for important intellectual content. M.S.A, M.E.A and G.A. provided administrative, technical, and material support; contributed to edited the manuscript and proofreading process; K.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Imai E, Haneda M, Yamasaki T. Effects of dual blockade of the renin-angiotensin system on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy and hypertension in the ORIENT a post-hoc analysis ORIENT-Hypertension. Hypertens Res 2013; 36: 1051-1059.
- Ruggenenti P, Fassi A, Ilieva AP, et Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004; 351: 1941-1951.
- Haller H, Ito S, Izzo JL, et al. ROADMAP Trial Investigators. Olmesartan for the delay or prevention of microalbuminuria in type 2 N Engl J Med. 2011; 364: 907-917.
- Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 N Engl J Med. 2001; 345: 870-878.
- Makino H, Haneda M, Babazono T, et INNOVATION Study Group. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 2007; 30: 1577-1578.
- Uzu T, Sawaguchi M, Maegawa H, et Reduction of microalbuminuria in patients with type 2 diabetes the Shiga Microalbuminuria Reduction Trial SMART. Diabetes Care 2007; 30: 1581-1583.
- Pham JT, Schmitt BP, Leehey DJ. Effects of Dual Blockade of the Renin-Angiotensin System in Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. J Nephrol Therapeutic. 2012; 2: 3.
- Chrysant SG, Chrysant Dual Renin-Angiotensin- Aldosterone Blockade Promises and Pitfalls. Curr Hypertens Rep. 2015; 17: 511.
- Chábova VC, Ä?ervenka The Dilemma of Dual Renin- Angiotensin System Blockade in Chronic Kidney Disease Why Beneficial in Animal Experiments But Not in the Clinic? Physiol Res. 2017; 66: 181-192.
- Grolla E, Bonanni L, Cutolo A, et Disputes in the Treatment of Diabetic Nephropathy The Dual Blockade of Renin-Angiotensin System. Exp Clin Endocrinol Diabetes. 2016; 124: 361-366.
- Elrggal ME, Ahmed SMS, Nahas Renin-Angiotensin- Aldosterone System Blockade in Diabetic Kidney Disease A Critical and Contrarian Point of View. Saudi J Kidney Dis Transpl 2016; 27: 1103-1113.
- Fried LF, Emanuele N, Zhang Combined Angiotensin Inhibition for the Treatment of Diabetic Nephropathy. N Engl J Med. 2013; 369: 1892-1903.
- Moher D, Liberati A, Tetzlaff J, et PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses the PRISMA statement. PLoS Med. 2009; 6: e1000097.
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001; 323: 42-46.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5 grading the strength of a body of evidence when comparing medical interventions agency for healthcare research and quality and the effective healthcare program. J Clin Epidemiol. 2010; 63: 513-523.
- Uzu T, Araki S, Kashiwagi A. Comparative Effects of Direct Renin Inhibitor and Angiotensin Receptor Blocker on Albuminuria in Hypertensive Patients with Type 2 Diabetes. A Randomized Controlled Trial. PLoS 2016; 11: e0164936.
- Kato S, Maruyama S, Makino H. Anti-albuminuric effects of spironolactone in patients with type 2 diabetic nephropathy a multicenter, randomized clinical trial. Clin Exp Nephrol. 2015; 19: 1098-1106.
- Van Buren PN, Adams-Huet B, Nguyen M. Potassium handling with dual renin-angiotensin system inhibition in diabetic Clin J Am Soc Nephrol. 2014; 9: 295-301.
- Kwakernaak AJ, Krikken JA, Binnenmars Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy a randomised clinical trial. Lancet Diabetes Endocrinol. 2014; 2: 385-395.
- Fernández-Juárez G, Luño J, Barrio V. 25 OH vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin angiotensin system. Clin J Am Soc 2013; 8: 1870-1876.
- Karalliedde J, Maltese G, Hill B. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc 2013; 8: 1899-1905.
- Mann JF, Anderson C, Gao P. Dual inhibition of the renin- angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens. 2013; 31: 414-421.
- Pruijm M, Hofmann L, Zanchi Blockade of the renin- angiotensin system and renal tissue oxygenation as measured with BOLD-MRI in patients with type 2 diabetes. Diabetes Res Clin Pract. 2013; 99: 136-144.
- Ghorbani A, Omidvar B, Beladi-Mousavi The effect of pentoxifylline on reduction of proteinuria among patients with type 2 diabetes under blockade of angiotensin system a double blind and randomized clinical trial. Nefrologia. 2012; 32: 790-796.
- Fernandez Juarez G, Luño J, Barrio V, et al. Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy a randomized trial. Am J Kidney 2013; 61: 211-218.
- Fallahzadeh MK, Dormanesh B, Sagheb MM. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy a randomized, double blind, placebo-controlled trial. Am J Kidney Dis. 2012; 60: 896-903.
- Rasi Hashemi S, Noshad H, Tabrizi A. Angiotensin receptor blocker and N acetyl cysteine for reduction of proteinuria in patients with type 2 diabetes mellitus. Iran J Kidney Dis. 2012; 6: 39-43.
- Kohan DE, Pritchett Y, Molitch M. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic J Am Soc Nephrol. 2011; 22: 763-772.
- de Zeeuw D, Agarwal R, Amdahl Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes VITAL study a randomised controlled trial. Lancet. 2010; 376: 1543-1551.
- Nakamura T, Fujiwara N, Sato E. Renoprotective Effects of Various Angiotensin II Receptor Blockers in Patients with Early- Stage Diabetic Nephropathy. Kidney Blood Press Res. 2010; 33: 213-220.
- Eyileten T, Sonmez A, Saglam M. Effect of renin-angiotensin- aldosterone system RAAS blockade on visfatin levels in diabetic Nephrology Carlton. 2010; 15: 225-229.
- Tan F, Mukherjee JJ, Lee Dual blockade of the renin angiotensin-aldosterone system is safe and effective in reducing albuminuria in Asian type 2 diabetic patients with nephropathy. Singapore Med J. 2010; 51: 151-156.
- Mehdi UF, Adams-Huet B, Raskin P. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic J Am Soc Nephrol. 2009; 20: 2641-2650.
- Krairittichai U, Chaisuvannarat V. Effects of dual blockade of renin-angiotensin system in type 2 diabetes mellitus patients with diabetic J Med Assoc Thai. 2009; 92: 611-617.
- Ogawa S, Mori T, Nako K. Combination therapy with renin- angiotensin system inhibitors and the calcium channel blocker azelnidipine decreases plasma inflammatory markers and urinary oxidative stress markers in patients with diabetic nephropathy. Hypertens Res. 2008; 31: 1147-1155.
- Galle J, Schwedhelm E, Pinnetti S. Antiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt Nephrol Dial Transplant. 2008; 23: 3174-3183.
- Wang G, Lai FM, Lai KB. Urinary messenger RNA expression of podocyte-associated molecules in patients with diabetic nephropathy treated by angiotensin-converting enzyme inhibitor and angiotensin receptor blocker. Eur J Endocrinol. 2008; 158: 317-322.
- Yoneda T, Takeda Y, Usukura M. Aldosterone breakthrough during angiotensin II receptor blockade in hypertensive patients with diabetes Am J Hypertens. 2007; 20: 1329-1333.
- Eijkelkamp WB, Zhang Z, Remuzzi G. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan RENAAL trial. J Am Soc Nephrol. 2007; 18: 1540-1546.
- Kurokawa K, Chan JC, Cooper Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes a subanalysis of Japanese patients from the RENAAL study. Clin Exp Nephrol. 2006; 10: 193-200.
- Stevanovic RD, Price DA, Lansang Renin release in response to Renin system blockade activation of the Renin system in type 1 diabetes mellitus. J Renin Angiotensin Aldosterone Syst. 2005; 6: 78-83.
- Song JH, Cha SH, Lee HJ. Effect of low-dose dual blockade of renin-angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney disease. Nephrol Dial Transplant. 2006; 21: 683-689.
- Matos JP, de Lourdes Rodrigues Effects of dual blockade of the renin angiotensin system in hypertensive type 2 diabetic patients with nephropathy. Clin Nephrol. 2005; 64: 180-189.
- Pohl MA, Blumenthal S, Cordonnier Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial clinical implications and limitations. J Am Soc Nephrol. 2005; 16: 3027-3037.
- Schjoedt KJ, Jacobsen P, Rossing Dual blockade of the renin- angiotensin-aldosterone system in diabetic nephropathy the role of aldosterone. Horm Metab Res. 2005; 37: 4-8.
- Schjoedt KJ, Andersen S, Rossing Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004; 47: 1936-1939.
- Chan JC, Wat NM, So Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL Study. Diabetes Care. 2004; 27: 874-879.
- Song JH, Lee SW, Suh JH. The effects of dual blockade of the renin angiotensin system on urinary protein and transforming growth factor-beta excretion in 2 groups of patients with IgA and diabetic Clin Nephrol. 2003; 60: 318-326.
- Rossing K, Jacobsen P, Pietraszek L. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy a randomized double-blind crossover Diabetes Care. 2003; 26: 2268-2274.
- Kim MJ, Song JH, Suh JH. Additive antiproteinuric effect of combination therapy with ACE inhibitor and angiotensin II receptor antagonist: differential short-term response between IgA nephropathy and diabetic Yonsei Med J. 2003; 44: 463-472.
- Jacobsen P, Andersen S, Rossing K. Dual blockade of the renin- angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003; 63: 1874-
- Jacobsen P, Andersen S, Jensen BR. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic J Am Soc Nephrol. 2003; 14: 992-999.
- Hollenberg NK, Price DA, Fisher Glomerular hemodynamics and the renin angiotensin system in patients with type 1 diabetes mellitus. Kidney Int. 2003; 63: 172-178.
- Jacobsen P, Andersen S, Rossing K. Dual blockade of the renin- angiotensin system in type 1 patients with diabetic nephropathy. Nephrol Dial 2002; 17: 1019-1024.
- Rossing K, Christensen PK, Jensen BR. Dual blockade of the renin-angiotensin system in diabetic nephropathy a randomized double-blind crossover Diabetes Care. 2002; 25: 95-100.
- Philips JC, Weekers L, Scheen AJ. Clinical study of the month. The CALM study assessing the combination of an angiotensin- converting enzyme inhibitor and an angiotensin II receptor antagonist in the treatment of diabetic nephropathy. Rev Med 2001; 56: 126-128.
- Andersen S, Tarnow L, Rossing P. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney 2000; 57: 601-606.
- Grzeszczak W, Zychma M, Zukowska-Szczechowska E, et Is PstI polymorphism of the angiotensin I converting enzyme gene associated with nephropathy development in non-insulin- dependent diabetes mellitus preliminary study. Pol Arch Med Wewn. 1997; 98: 19-25.
- Strojek K, Grzeszczak W, Lacka Activity of the renin- angiotensin-aldosterone system in euglycemic type I diabetic patients on intensive insulin treatment without diabetic neuropathy. Pol Arch Med Wewn. 1995; 94: 208-213.