A Rare Case of Recurrent Caesarean Scar Ectopic Pregnancy after Wedge Resection of Caesarean Scar Ectopic Pregnancy
Author'(s): Lou Ying Yiing, Boret Tony, and Awala Alero
Department of Obstetrics & Gynaecology, Watford General Hospital, Watford, United Kingdom
*Correspondence:
Ying Yiing Lou, Department of Obstetrics & Gynaecology, Watford General Hospital, Vicarage Road, Watford, United Kingdom, Tel: 0044 (0) 1923217935; E-mail: yingyiing.lou@nhs.net.
Received: 29 July 2017 Accepted: 27 August 2017
Citation: Lou Ying Yiing, Boret Tony, Awala Alero. A Rare Case of Recurrent Caesarean Scar Ectopic Pregnancy after Wedge Resection of Caesarean Scar Ectopic Pregnancy. Gynecol Reprod Health. 2017; 1(2): 1-4.
Abstract
The implantation of a pregnancy within the scar of a previous caesarean delivery is the rarest of ectopic pregnancy location. If it is diagnosed early, treatment options are capable of preserving the uterus and subsequent fertility. Diagnosis involves a combination of clinical symptoms, serology and ultrasound. Management of caesarean scar ectopic pregnancy (CSEP) may involve medical or surgical treatment, or a combination. We reported a rare case recurrent CSEP after wedge resection of CSEP on a 31-year-old para 1 with previous caesarean section who was at 5 weeks of gestation. She underwent a combined surgical management with suction curettage under ultrasound guidance and medical treatment with methotrexate.
Keywords
Introduction
Although caesarean section is a common operation, caesarean scar ectopic pregnancy (CSEP) is comparatively a rare occurrence with an incidence of 1:1800-1:2500 [1]. This is likely to increase with the increasing caesarean rate worldwide. Every healthcare professional managing early pregnancy complications should be vigilant in the possibility of a CSEP. Without a high index of suspicion and correct early diagnosis, like other ectopic pregnancies, this abnormal implantation can lead to placenta percreta/acccreta, uterine rupture and/or hysterectomy, with consequent maternal morbidity and loss of future fertility. We review the available management options of CSEPs, as well as review the existing literature regarding the future risk of recurrence and their future fertility after surgical resection of CSEP.
Case report
A 31-year-old gravida 3 para 1, who had previous caesarean section 5 years ago, presented with 3-day-history of PV bleeding at 5 weeks of gestation. She had an open wedge resection to remove CSEP 6 months earlier in Romania.
She was haemodynamically stable. On examination, her abdomen was soft and non-tender. Her cervix was closed, there was mild bleeding and there was no cervical motion tenderness. Her haemoglobin was 126g/L, serum hCG was 14,447U/L and progesterone was 96.3nmol/L.
A transvaginal ultrasound scan reported an anteverted uterus with thickened endometrium at 12.6mm. There was a gestational sac with mean sac diameter 10.9mm which contained 2 yolk sacs with no fetal pole seen partially implanted within previous caesarean section scar (Figure 1). The myometrium anterior to the sac was noted to be 2.44mm in the anterior-posterior dimension (Figure 2). There was no evidence of free fluid. These findings represent a CSEP.
Following the consultation, patient was offered combined surgical treatment with suction curettage and medical treatment with methotrexate. Patient had initially undergone ultrasound guided suction curettage. Intrauterine balloon tamponade by Foley catheter size 30 was used for 18 hours. Anti-D was given as patient was rhesus negative. She then received the methotrexate injection the following day prior to discharge. She was followed up on day 4, day 7 and day 14 after her methotrexate treatment and her hCG had dropped down to 39U/L (Graph 1). Histology has confirmed product of conception with non-molar chorionic villi. Her urine pregnancy test was negative a week later.
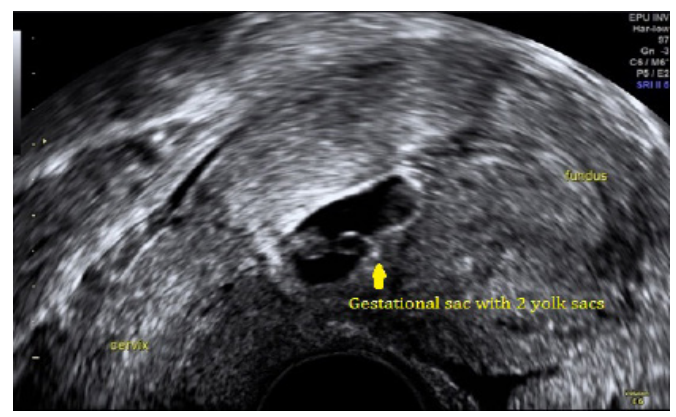
Figure 1: Shows a gestational sac contains 2 yolk sacs partially implanted within previous caesarean section scar.
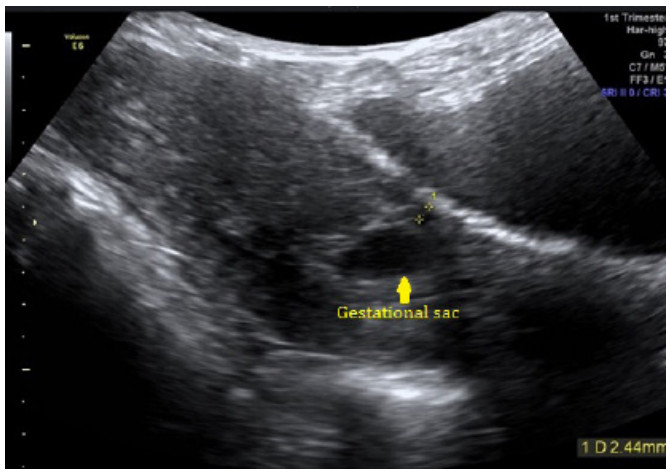
Figure 2: Shows the myometrium anterior to the sac was noted to be 2.44mm in the anterior-posterior dimension.
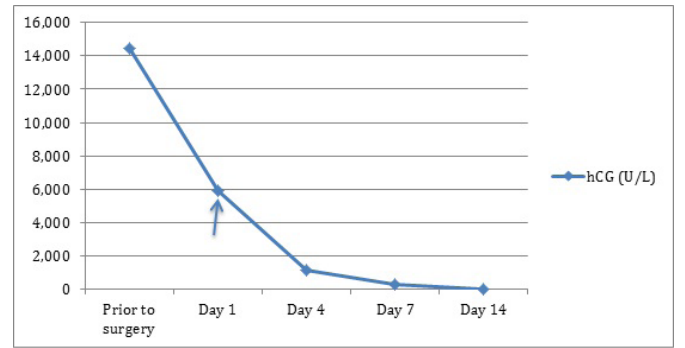
Graph 1: hCG levels prior to and after surgery. Methotrexate was administered on Day 1 post operation.
Discussion
The exact cause of implantation of the gestation into the scar of a previous caesarean section is not well understood. It was speculated that CSEP results from implantation through a microscopic dehiscence tract between the endometrial cavity and the scar [2].
Vial et al. proposed two different types of CSEP. The first occurs due to the implantation of the gestational sac on the scar with progression towards either the cervicoisthmic space or uterine cavity and the second as a result of a deep implantation into uterine scar with progression towards the serosal surface [3]. While the first type of pregnancy may result in a viable birth, it has an increased risk of life threatening bleeding from the implantation site. The second generally leads to a rupture and bleeding during the first trimester [3].
CSEP may present from as early as 5-6 weeks (4) to as late as 16 weeks [5]. Painless vaginal bleeding is the most common presenting symptom of caesarean scar pregnancy [6]. Severe acute pain with profuse bleeding implies an impending rupture.
Ultrasound is the first line diagnostic tool for CSEP. A sagittal view along the long axis of the uterus through the gestation sac can localise a CSEP with confidence. Jurkovic et al. used the following criteria to diagnose early caesarean scar pregnancies by transvaginal sonography (TVS) [1].
- The uterine cavity is empty.
- The gestational sac is located anteriorly at the level of the internal os, covering the visible or presumed site of the caesarean section scar.
- Doppler study suggests a functional placental circulation defined by increased vascularity by colour flow evaluation, a peak velocity greater than 20 cm/s, and a pulsatility index of less than 1.
- There is no ‘‘sliding organs sign,’’ defined as the inability to displace the gestational sac with gentle pressure applied by the transvaginal probe.
Because of the rarity of the condition, majority of CSEPs are case reports or small case series reported in the literature, with no consensus on the preferred mode of treatment. Generally, termination of pregnancy in the first trimester is strongly recommended, as there is a high risk of subsequent uterine rupture, massive bleeding and life-threatening complications. Both medical and surgical approaches have proven successful.
CSEPs have been shown to respond well to Methotrexate (MXT) (dose of 50 mg/m2), especially in those with hCG levels <5000 U/L [7]. Conservative medical treatment is appropriate for a woman who is pain free and haemodynamically stable with an unruptured CSEP of <8 weeks of gestation and a myometrial thickness <2 mm between the CSEP and bladder [8].
Blind uterine curettage as a primary treatment for CSEP is therefore insufficient and should be discouraged. But some authors argued for suction curettage under ultrasound guidance in selected cases where the gestation is ≤ 7 weeks and the myometrial thickness anterior to the CSEP is ≤ 3.5 mm [9]. Notwithstanding this, the lack of direct visualisation, risk of a local haematoma formation and the need for a prolonged hCG follow up remain the major drawbacks and more reports are needed to rationalise this treatment modality [9].
Various haemostatic measures have been used successfully as an adjunct to conservative treatment of viable CSEPs for the prevention and control of profuse bleeding, e.g. local injection of vasopressin [10], intrauterine balloon tamponade by Foley catheter (30–90 ml of balloon capacity for 12–24 hours) [10], Shirodkar suture [11], selective bilateral uterine artery embolisation [12] and bilateral uterine artery ligation [13].
Combined medical treatment in varying regimens has been described by many authors. Various sequences of combination have been described:
- Local potassium chloride →TVS guided sac aspiration →local MTX injection →intramuscular MTX injection [14].
- Systemic MTX →TVS guided sac aspiration [15].
- Sac aspiration (transvaginal or transabdominal) → local MTX injection [3].
- Sac aspiration under ultrasound guidance →systemic MTX [16].
- Systemic MTX →sac aspiration by vaginal route/local MTX [17].
We had chosen surgical management with suction curettage with the benefit of leading to more rapid resolution of hCG as compared to medical treatment combined with medical treatment with MTX as persistent trophoblastic tissue may occur after surgical management. It has been reported that suction curettage with MTX can be considered an effective treatment option with good maternal outcomes [18]. This has reflected in our case by choosing a combined surgical with medical treatment and the hCG has dropped down from 14,447 U/L to 39U/L within 2 weeks of treatment.
Transabdominal excision of these lesions by wedge resection has been described by laparotomy or robotic-assisted laparoscopy [19]. Resection also allows for revision of the lower uterine segment which theoretically may reduce risk for recurrence [20]. However, owing to the insufficient data, it is difficult to advise our patient on the future risk of recurrence.
Likewise, no reliable statistics exist on the safe interval between surgical resection of CSEP and any risk to a subsequent pregnancy. Although there is little data to suggest any danger in conceiving soon after treatment with MTX, some women may prefer to wait at least 6 months to minimise any potential teratogenic effect in a new pregnancy [21]. Despite a 10-hour half-life, MTX can still be found in the liver and kidneys months later [22]. Some authors recommend future pregnancies be avoided for more than 3 months and probably 1 or 2 years [3]. We had advised our patient not to conceive 3 months following the methotrexate treatment.
Senior clinicians must be involved in patient counselling and making appropriate management plan and follow up. Owing to the lack of reliable data on the best evidence, women should be given information on the various treatment options available to date to enable them to make an informed choice.
Summary
Increasing caesarean section rates imply that clinicians will encounter CSEP from time to time. Prompt and accurate diagnosis of CSEP and individualised treatment and follow up are required to reduce overall morbidity.
Declaration of conflicting interest
The authors have no conflicts of interest (political, personal, religious, ideological, academic, intellectual, commercial or any other) to declare in relation to this manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit organisation.
References
- Jurkovic D, Hillaby K, Woelfer B, et First-trimester diagnosis and management of pregnancies implanted into the lower uterine caesarean section scar. Ultrasound Obstet Gynecol. 2003; 21: 220-227.
- Fylstra Ectopic pregnancy within a cesarean scar: a review. Obstet Gynecol Surv. 2002; 57: 537-543.
- Vial Y, Petignat P, Hohlfeld Pregnancy in a Caesarian scar. Ultrasound Obstet Gynecol. 2000; 16: 592-593.
- Seow K-M, Huang L-W, Lin YH, et Caesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004; 23: 247-253.
- Smith A, Maxwell D, Ash Sonographic diagnosis of caesarean scar pregnancy at 16 weeks. J Clin Ultrasound.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis and Obstet Gynecol. 2006; 107: 1373-1377.
- Donnez J, Godin P-A, Bassil Successful methotrexate treatment of a viable pregnancy within a thin uterine scar Br J Obstet Gynaecol. 1997; 104: 1216-1217.
- Maymon R, Halperin R, Mendlovic S, et Ectopic pregnancies in a caesarean scar: review of the medical approach to an iatrogenic complication. Hum Reprod Update. 2004; 10: 515-523.
- Arslan M, Pata O, Dilek TU, et Treatment of viable cesarean scar ectopic pregnancy with suction curettage. Int J Gynecol Obstet. 2005; 89: 163-166.
- Chuang J, Seow KM, Cheng WC, et Conservative treatment of ectopic pregnancy in a caesarean section scar. BJOG. 2003; 110: 869-870.
- Ben Nagi J, Ofili-Yebovi D, Sawyer E, et al. Successful treatment of a recurrent Caesarean scar ectopic pregnancy by surgical repair of the uterine defect. Ultrasound Obstet 2006; 28: 855-857.
- Ghezzi F, Lagana D, Franchi M, et Conservative treatment by chemotherapy and uterine arteries embolization of a cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002; 103: 88-91.
- Sum TK, Wong SH, Tai CM, et An ectopic pregnancy in a previous caesarean section scar: treatment with systemic methotrexate and uterine artery embolisation. J Obstet Gynecol. 2000; 20: 328.
- Tan G, Chong YS, Biswas A. Caesarean scar pregnancy: a diagnosis to consider carefully in patients with risk factors. Ann Acad Med 2005; 34: 216-219.
- Ravhon A, Ben-Chetrit A, Rabinowitz R, et al. Successful methotrexate treatment of a viable pregnancy within a thin uterine Br J Obstet Gynaecol. 1997; 104: 628-629.
- Wang W, Long W, Yu Q. Complication of Cesarean section: pregnancy on the cicatrix of a previous cesarean Chin Med J. 2002; 115: 242-246.
- Hwu Y-M, Hsu C-Y, Yang H-Y. Conservative treatment of caesarean scar pregnancy with transvaginal needle aspiration of the embryo. BJOG. 2005; 112: 841-842.
- Datta S, Jha C. Suction evacuation with methotrexate as a successful treatment modality for caesarean scar Case series. Sultan Qaboos Univ Med J. 2015; 15: e539-e545.
- Wang G, Liu X, Bi F, et al. Evaluation of the efficacy of laparoscopic resection for the management of exogenous cesarean scar Fertil Steril. 2014; 101: 1501-1507.
- Siedhoff MT, Schiff LD, Moulder JK, et Robotic- assisted laparoscopic removal of cesarean scar ectopic and hysterotomy revision. Am J Obstet Gynecol. 2015; 212: 681.
- Nawroth F, Foth D, Wilhelm L, et al. Conservative treatment of ectopic pregnancy in a cesarean section scar with methotrexate: a case report. Eur J Obstet Gynecol Reprod 2001; 99: 135-137.
- Feldenkamp M, Carey Clinical teratology counselling and consultation case report. Teratology. 1993; 7: 533-539.