Acute Fulminant Chlamydia pneumoniae Myocarditis Treated with Mechanical Circulatory Support in a Female Adult: A Case Report
Author'(s): Maša BiberiÄ?1,4*, Siniša Zrna1,4, Jurica JuraniÄ?1, Vlatka Sotošek TokmadžiÄ?1,2, Branka KurtoviÄ?3 and željko župan1,2
1 Departemnt of Anesthesiology and Intensive Care Medicine, Clinical Hospital Center Rijeka, Rijeka, Croatia.
2 Department of Anesthesiology, Reanimatology and Intensive Care Medicine, Faculty of Medicine, University of Rijeka, Rijeka,Croatia.
3 Department of Surgery, Division of Cardiac Surgery, Clinical Hospital Center Rijeka, Rijeka, Croatia.
4 Department of Anesthesiology, Reanimatology and Intensive Care Medicine, General Hospital Pula, Pula, Croatia.
*Correspondence:
Maša BiberiÄ?, Resident of Anesthesiology, Clinical Hospital Center Rijeka, Rijeka, Croatia, E-mail: masa.biberic@gmail.com.
Received: 06 May 2018; Accepted: 18 June 2018
Citation: Maša BiberiÄ?, Siniša Zrna, Jurica JuraniÄ?, et al. Acute Fulminant Chlamydia pneumoniae Myocarditis Treated with Mechanical Circulatory Support in a Female Adult: A Case Report. Anesth Pain Res. 2018; 2(1): 1-5.
Abstract
Acute fulminant myocarditis (AFM) is characterized by sudden onset of severe heart failure or cardiogenic shock. In this paper we describe a case of acute fulminant myocarditis and chronic constrictive pericarditis due to Chlamydia pneumoniae (C. pneumoniae) infection and the use of mechanical circulatory support (MCS) for the treatment of refractory cardiogenic shock. Acute fulminant myocarditis and chronic C. pneumoniae constrictive pericarditis led
to a sudden onset of circulatory arrest and progressive cardiogenic shock in this particular case. The diagnosis was confirmed with transfemoral biopsy of the heart muscle and a positive IgG/IgM-enzyme-linked immunosorbent assay (ELISA)-serum test. After the specific antimicrobial treatment combined with MCS the patient fully recovered and was discharged from the hospital.
Keywords
Introduction
Chlamydia pneumoniae (C. pneumoniae) is a common chlamydial species causing infections in humans. C. pneumoniae is often involved in acute respiratory infections in all age groups [1-3]. It is an obligate, gram negative, intracellular bacteria. Studies of C. pneumoniae infection published so far have found association of the organism with 6%-22% of all lower respiratory tract infections [4]. Pneumonia, bronchitis and pharyngitis are the most common infections caused by C. pneumoniae. It is transmitted from human to human by respiratory tract secretions; many infected people, however, are just transmitters of the infection and people with asymptomatic infections play a role in the spread of this pathogen [5].
Infections may be severe if there are some underlying diseases and complications. C. pneumoniae may cause inflammatory heart disease, but this seems to be unusual in clinical practice [6].
Acute fulminant myocarditis is a very rare complication of C. pneumoniae infection. There might be some individual host factors that make certain individuals more prone to develop myocarditis, perimyocarditis, or endocarditis when infected by C. pneumoniae [7]. A minority of C. pneumoniae myocarditis may develop dilated cardiomyopathy and patients may present acute cardiac failure. There are very few clinical publications about C. pneumoniae as a cause of myocarditis and pericarditis, especially with the use of MCS as means of treatment [8].
A report involving C. pneumoniae myocarditis that was supported with intra-aortic balloon pump was shown by Pao-Tsuan Kao et al. [9]. Serological testing is the main method to diagnose the infection [10]. Significant limitations influence the accurate diagnosis of a C. pneumoniae infection. Among them is the lack of existence of standardized and commercially available tests, and the wide variations of diagnostic criteria used in the studies are not of help in this context.
Written consent for publishing the article was signed by the patient.
Case Report
A previously healthy 47-year old female with symptoms of joint pain, vomiting, general weakness, dizziness, hair loss and weight loss in the last two months was admitted to Clinical Hospital Center Rijeka, Rijeka, Croatia. Initial clinical examination revealed blood pressure of 85/65 mm Hg, electrocardiogram (ECG) showed a Mobitz II second-degree atrioventricular (AV) block with the ventricular rate of 63 beats per minute (bpm). Initial chest and abdominal X-ray were also performed. The chest X-ray showed a possible heart enlargement, while the abdominal X-ray was clean. An initial blood test was also performed and showed anemia (hemoglobin blood level of 105 g/L, hematocrit of 0.326) and an elevated C-reactive protein (CRP) concentration of 139.0 mg/L, with normal white blood cell count number (WBC) of 5.3x109/L. Other blood parameters were in range or only slightly altered.
During the diagnostic processing at the Emergency Department the patient went into a circulatory arrest. Orotracheal intubation was performed and cardiopulmonary resuscitation (CPR) was initiated immediately. The starting rhythm was ventricular fibrillation. The patient was defibrillated immediately, but the rhythm changed to asystole. CPR was continued following the Advanced Life Support (ALS) protocol, which resulted with return of spontaneous circulation (ROSC).
The patient was transferred to the Intensive Cardiology Department, Clinical Hospital Center Rijeka, Rijeka, Croatia, where initial transesophageal echocardiography (TEE) showed akinesis of the middle and apical segment of the anterior, anteroseptal, inferoseptal, inferior and inferolateral myocardial wall (left ventricular internal diameter end diastolic dimension was 54 mm, left ventricular internal diameter end systolic 40 mm, interventricular septum 9 mm, left atrium 42 mm, right ventricle 23 mm). It showed hypokinesis of the basal segments of all myocardial walls. The Color Doppler ultrasonography showed moderate/severe mitral regurgitation, severe tricuspid regurgitation and indirect signs of increased pressure in pulmonary circulation. The estimated ejection fraction was 15-20%. The coronarography showed normal findings of coronary arteries.
The patient was initially hemodynamically stabilized with norepinephrine at infusion rate of 0.3 μg/kg/min (Arterenol, Sanofi Aventis, Paris, France and B Braun Medical INC infusion pump, Melsungen, Germany). Inotropic cardiac support was initiated with dobutamine 2.5 μg/kg/min (Dobutamin Admeda, Nienwohld, Germany and B Braun Medical INC infusion pump, Melsungen, Germany) and levosimendan 0.1 μcg/kg/min (Simdax, Orion Pharma, Ljubljana, Slovenia and B Braun Medical INC infusion pump, Melsungen, Germany). Ceftriaxone 2g intravenous (i.v.) (Lendacin, Sandoz GmbH, Camberley Surrey, United Kingdom) was initially given as empirical antibiotic treatment. With the initial therapy, the patient was hemodynamically stabilized with mildly elevated blood lactate concentration of 2.0 mmol/L and adequate urine output.
On the third day of treatment at Cardiology Intensive Care Unit the patient started to deteriorate hemodynamically, showing persistent hypotension refractory to medication and volume resuscitation and was transferred to the Cardiac Surgery Intensive Care Unit (ICU). Upon admission at the ICU, the patient was already intubated and mechanically ventilated with signs and symptoms of severe heart failure, hemodynamically unstable despite the application of vasoactive and inotropic drugs.
The patient was sedated with midazolam 6 mcg/kg/min (Midazolam, B Braun, Melsungen, Germany and B Braun Medical INC infusion pump, Melsungen, Germany) and sufentanyl 0.01 mcg/kg/min (Sufentanyl, Renaudin, Itxassou, France and B Braun Medical INC infusion pump, Melsungen, Germany). Inotropic and vasoactive support with norepinephrine at dose of 0.6 mcg/kg/min (Arterenol, Sanofi Aventis, Paris, France and B Braun Medical INC infusion pump, Melsungen, Germany) and dobutamine 2.4 mcg/kg/min (Dobutamin, Admeda, Nienwohld, Germany and B Braun Medical INC infusion pump, Melsungen, Germany) were continued, and additionally enoximone 7 mcg/kg/min (Perfan, Carinopharm GmbH, Elze, Germany and B Braun Medical INC infusion pump, Melsungen, Germany) was initiated. The patient continued to be hemodynamically unstable with MAP of 60 mmHg, whereas the ECG showed atrial fibrillation with a ventricular rate of 120 bpm.
At this point, the antiarrhythmic drug amiodarone (Cordarone, Sanofi Aventis, Paris, France and B Braun Medical INC infusion pump, Melsungen, Germany) was included in the therapy, at 12 mcg/kg/min. The diuresis was held above 1 mL/kg/h. Laboratory tests showed high anaerobic metabolism indices (table 1) with a lactate concentration of 5.3 mmol/L, while the blood count was in range. The glucose level was kept in range (8-12 mmol/L) with conventional insulin therapy. The patient’s body temperature was from 37.5°C to 38.4°C, with normal total number of WBC (6.9x 10°/L), concentration of CRP 147 mg/L and procalcitonin (PCT) level of 1.3 ng/mL. The empirical antibiotic therapy was changed to meropenem 3 x 2 g i.v. (Meropenem Belupo 500 mg, Belupo, Koprivnica, Croatia) and teicoplanin 1 x 200 mg i.v. (Targocid 400 mg, Sanofi Aventis, Paris, France).
The initial creatine kinase (CK) level was 1424 U/L, N-terminal proBrain natriuretic peptide (NT-proBNP) 50601 ng/L and Troponin T (s) 140 ng/L. Enteral feeding was started through nasogastric tube with 50 mL/h/16 h (Fresubin HP Energy, Fresenius Kabi, Bad Homburg, Germany, 1.5 kcal/mL) and 20 mL/h/16 h of sweet tea due to the patient’s needs. A transthoracic echocardiography (TTE) was performed, which showed severe global hypokinesis with a global ejection fraction of less than 10%, dilatation of both ventricles and severe mitral and tricuspid regurgitation. The initial chest X-ray in ICU was without any special features. The diagnosis established that the patient had a severe acute infective myocarditis of unclear etiology.
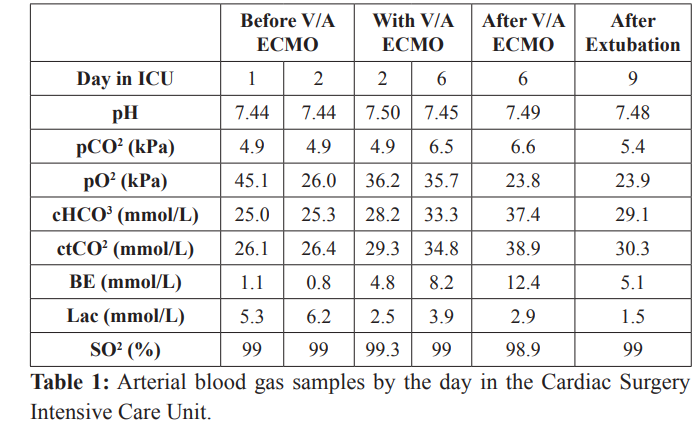
On the second day of her admission at the ICU, the concentration of lactate was 6.2 mmol/L (Table 1) with no change in TEE findings. Despite all the measures taken there had been no hemodynamical improvements. In agreement with cardiac surgeons it was decided to initiate cardiopulmonary support by peripheral venoarterial extracorporeal membrane oxygenation (peripheral V/A ECMO). The patient’s right femoral vein and right subclavian artery were surgically cannulated. Rotaflow console (Maquet, Hirrlingen, Germany) with Maquet oxygenator (Bioline coated tubing set with PLS-i Oxygenator, Bioline, Coimbatore, India) was used. A venous cannula (2155 HLS Cannulae Maquet, Hirrlingen, Germany, with Bioline Coating, Bioline, Coimbatore, India, length 55 cm) was set in the right femoral vein and an arterial cannula (Edwards Lifesciences Reasearch Medical.Inc, Irvine, CA, USA, length 15 cm, diameter 24 fr) was set in the right subclavian artery with surgical access. The flow was established considering the predicted cardiac index (2.4 l/m2 x body surface area- BSA, maximal peripheral V/A ECMO-derived blood flow for the first 24 hours, 100 % of predicted cardiac index). Soon after cannulation of the subclavian artery the patient’s right arm had swelled and showed signs of venous congestion. Surgical exploration of the right subclavian region showed a regional hematoma which was evacuated. The place of cannulation was eventually changed to the right femoral artery (1715 arterial HLS Cannulae, Maquet, Hirrlingen, Germany, with Bioline Coating, Bioline, Coimbatore, India, length 15 cm) with reperfusion cannula (Medtronic, Harleen, The Netherlands, diameter 7 fr) for the distal perfusion of the cannulated leg [11].
The patient’s activated clotting time (ACT) and activated partial thromboplastin time (aPTT) were measured (ACT Plus Automated Coagulation Time System, Medtronic, Heerlen, The Netherlands) every four to six hours and kept in range. Target ACT level was 120-200 s and aPTT 1.5-3 times that of the normal level. A continuous infusion of heparin was started only to the reperfusion cannula according to the level of aPTT (0.7-1.2 IU/kg/h) (Heparin, Panpharma, Luitre, France and B Braun Medical INC infusion pump, Melsungen, Germany). After initiation of peripheral V/A ECMO, the infusion of dobutamine was discontinued, whereas the infusion of enoximone and norepinephrine was reduced (enoximone 1.1 mcg/kg/min, norepinephrine 0.1 mcg/kg/min and B Braun Medical INC infusion pump, Melsungen, Germany).The first arterial blood gas (ABG) samples after initiation of peripheral V/A ECMO showed decrease in lactate concentration (Table 1). A positive fluid balance was achieved during the first 24 hours of MCS support. In order to maintain diuresis above 1.5 mL/kg/h, infusion of furosemide (Furosemid, Belupo, Koprivnica, Croatia) was started, at 1.2 mcg/kg/min. Anemia was corrected with two units of red blood cells. Atrial fibrillation was converted to sinus tachycardia with a frequency around 110 bpm and amiodarone was discontinued.
On the fourth day after admission, a transfemoral biopsy of the heart muscle and pericardium was made. Diagnosis was confirmed with the findings that correspond to lymphocytic myocarditis and chronic pericarditis. Immunoglobulins (Octagam, Jana Pharm, Zagreb, Croatia) 25 g per day in combination with corticosteroids metilprednisolone (Solu-Medrol, Pfizer, Brussels, Belgium) 250 mg per day were added to the therapy. The tracheal aspirate was positive to Candida albicans with 1000 colony-forming unit (CFU), which was determined to be as contamination.
The blood flow of peripheral V/A ECMO was gradually decreased until the sixth day of the ICU bed rest (70 % of predicted cardiac index) and it was well tolerated. With a flow below 70 % of predicted cardiac index, the patient was still hemodynamically unstable. In agreement with cardiac surgeons, the patient underwent median sternotomy and exploration of mediastinum was made. A severe form of constrictive pericarditis was found. Pericardiotomy and adhesiolisis were made. The intraoperative TEE performed after the pericardiotomy showed a significant improvement of the myocardial function with a global ejection fraction of 30 % (Figure 1). The peripheral V/A ECMO was successfully removed (after 96 hours in total). Two units of blood were necessary postoperatively to correct anemia with a hemoglobin level of 74 g/L and hematocrit 0.25 before blood administration. After peripheral V/A ECMO removal lactate concentration was 2.9 mmol/L (Table 1). Norepinephrine was completely discontinued while enoximone was continued in lower doses of 1 mcg/kg/min combined with dobutamine 2 mcg/kg/min for two more days.
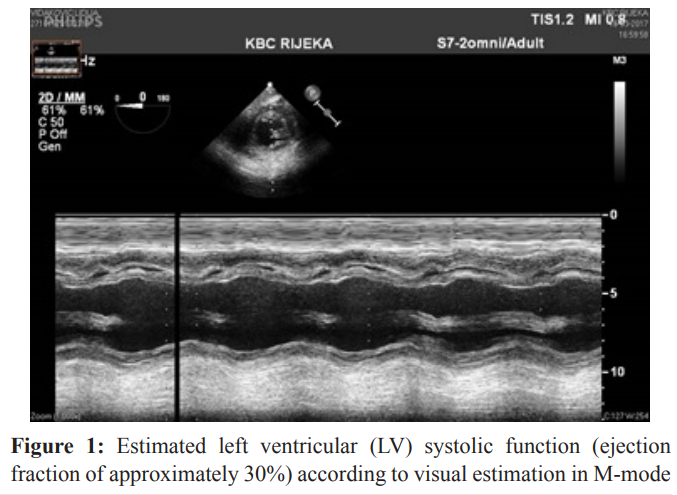
of transgastric, mid-papillary view.
Because of low concentration of CRP of 5.7 mg/L and WBC number of 9.8 x 10°/L, procalcitonine 0,379 ng/mL in combination with fever 38°C, at the ninth day of ICU treatment antibiotic therapy was empirically changed from meropenem and teicoplanin to colistin (Colistin Alvogen, Xellia Pharmaceuticals APS, Copenhagen, Denmark) 3 x 1000000 international units (IU) and fluconazole (Diflucan, Pfizer, Brusseles, Belgium) 1 x 200 mg.
With a wide range of tests we excluded sepsis (multiple hemoculture tests), urinary tract infection (multiple urine culture tests), central nervous system infection (cerebrospinal fluid culture test and cerebrospinal fluid-CSF-antigen-Latex agglutination test for Neisseria meningitidis, Escherichia coli, Beta Hemolytic Streptococcus group B (BHS-B), Streptococcus pneumoniae, Haemophilus influenzae), influenza A and B infection (Influenza A and B complement fixation test), Mycoplasma pneumoniae infection (Mycoplasma pneumoniae complement fixation test), Epstein-Barr virus infection (EBV) (EBV IgG/IgM-ELISA- serum test), varicella-zoster virus infection (VZV) (VZV IgG/ IgM-ELISA- serum test), cytomegalovirus infection (CMV) (CMV IgG/IgM-ELISA-serum test, CMV DNA PCR-urine and CMV DNA PCR-plasma, method LightCycler 2.0 CMV Quant Kit-GeneProof), respiratory syncytial virus infection (RSV) (RSV complement fixation test), parvo B 19 virus infection (PARVO B19 IgG/IgM-ELISA- serum test), herpes simplex virus (HSV) 1 infection (HSV 1 IgG/IgM-ELISA-serum test), herpes simplex virus 2 infection (HSV 2 IgG/IgM-ELISA-serum test), rotavirus infection (Rota-adenovirus antigen test), Clostridium difficile alimentary tract infection (Clostridium difficile-toxin A/B test), Lyme boreliosis infection (Borelia burgdorferi IgG/IgM-enzyme- linked fluorescent assay (ELFA)-CSF test), and brucelosis infection (Rose Bengal test). The only positive test was Chlamydia pneumoniae (IgG/IgM-ELISA-serum test), IgM (20,1 U) and IgG (50,0 U) antibody positive. Immediately upon the arrival of the positive finding on the tenth day of stay in ICU, specific antibiotic therapy with doxycycline (Hiramicin, Pliva Croatia) 2 x 100 mg was started.
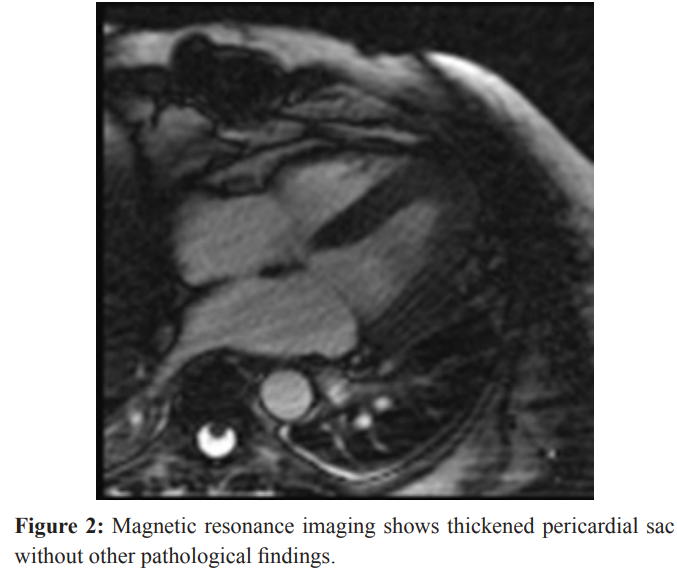
Magnetic resonance imaging (MRI) (Siemens Magnetom Avanto 1,5 Tesla, Munich, Germany) of the heart was made on the sixteenth day of stay in ICU and it showed ejection fraction of 71% (end-diastolic volume-EDV 141.5 mL, end-systolic volume- ESV 41 mL, stroke volume-SV 100.4 mL), thickened pericardiac sac without segmental contractility distortion or other pathological findings. After the application of contrast diffuse increase in myocardial signal was seen which points to the inflammatory etiology of diffuse miopericarditis (Figure 2).
The patient successfully weaned from ventilator and was extubated at the ninth day of stay in the ICU. Newly formed quadriplegia of unknown cause has been noticed. Computed tomography (CT) (Siemens SOMATOM definition AS, Forcheim, Germany) scan of the brain was made and did not show any abnormalities or ischemic lesions. Electromyography (EMG) (Nihon Kohden, Tokyo, Japan) showed damaged motor neurons and a working diagnosis of critical illness polyneuropathy was made. Acute neurological event was excluded and the patient was transferred to the Immunology Department for further care and treatment after twenty four days of treatment in ICU. After several months of physical therapy the quadriplegia gradually withdrew.
Discussion
Myocarditis can be caused by many different infectious and noninfectious entities. The most common causes of myocarditis are viral infections, followed by bacterial infections, medications, and autoimmune disorders [12]. A wide range of bacterial species are known to cause myocarditis, such as Corynebacterium diptheriae, Staphylococcus aureus and Borrelia burgdorferi. Chlamydiae are recognized as very rare pathogens that can cause myocarditis [13]. Chlamydiae are known as common human pathogens with a tendency to cause infections with minimal to no symptoms, but in immunocompromized patients can cause severe infections [14].C. pneumoniae is an obligate intracellular bacteria with a unique biphasic developmental cycle [15]. It is a small gram negative bacterium (0.2-1um) and is spread by aerosolized respiratory secretions [9]. The most common clinical manifestations of C. pneumoniae infection are pneumonia, bronchitis or pharyngitis, through persistent epithelial infection. Studies have shown that chlamyidae are able to infect neonate rats myocytes and cause significant damage to the cells by producing reactive oxygen species [16]. Culture is highly specific but demanding and expensive to create. Serology is the method of choice in diagnosing this specific pathogen [17]. There is ongoing research with association between C. pneumoniae infection and development of atherosclerosis [18].
In this report we described a very rare case of fulminant peri/ myocarditis in a previously healthy patient who presented acute- onset heart failure. The reason of such severe clinical manifestation is unclear. A possible explanation for such a dramatic clinical presentation could be a very infectious species of bacteria, or it could be assigned to unrecognized immunodeficiency in the patient.
Due to the severity of infection acute hearth failure occurred, probably due to fulminant systemic inflammation which led to the release of numerous proinflammatory mediators (cytokines, chemokines) leading to depression of the heart function. This clinical presentation required rapid intervention. Initial medical treatment, which was applied immediately after admission, failed and a decision for MCS with peripheral V/A ECMO as a “bridge to recovery“ was made. A highly likely fatal outcome was avoided and the patient was ultimately discharged from hospital. To the best of our knowledge this is the first clinical report of C. pneumoniae infection with a clinical presentation of fulminant peri/myocarditis with ECMO support that showed a favorable outcome.
Conclusion
In conclusion, C. pneumoniae carditis can clinically, in rare cases, present with fulminant myocarditis. C. pneumonaie should be considered in patients with unexpected cardiac symptomatology. MCS should be initiated in the early stages of profound circulatory collapse resistant to pharmacological treatment. Early start of MCS support can contribute to rapid recovery of hemodynamic stability and faster recovery of cardiac function and adding significantly higher odds of survival.
References
1.Nieto FJ, Folsom AR, Sorlie PD, et al. Chlamydia pneumoniae Infection and Incident Coronary Heart Disease. American Journal of Epidemiology. 1999; 150: 149-156.
2.Saikku P, Wang SP, Kleemola M, et al. An epidemic of mild pneumonia due to an unusual strain of Chlamydia psittaci. J Infect Dis. 1985; 151: 832-839.
3.Grayson JT, Kuo CC, Wang SP, et al. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory infections. N Engl J Med. 1986; 315: 161-168.
4.Hammerschlag M. Chlamydia pneumoniae and the lung. Eur Respir J. 2000; 16: 1001-1007.
5.Grayston JT. Infections Caused by Chlamydia pneumoniae strain TWAR. Clinical Infectious Disease. 1992; 15: 757-763.
6.Gnarpe H, Gnarpe J, Gästrin B, et al. Chlamydia pneumoniae and myocarditis. Scand J Infect Dis. 1997; 104: 50-52.
7.Gnarpe HA, Gnarpe JA. Chlamydia pneumoniae. Infect Dis. 2004: 187-197.
8.Kuo CC, Jackson LA, Campbell LA, et al. Chlamydia pneumoniae (TWAR). Clinical Microbiology Reviews. 1995; 8: 451-461.
9.Pao-Tsuan Kao, Chin-Yen Chiang. Fulminant Myocarditis due to Chlamydia pneumoniaeComplicated with Peripheral Gangrene and Cerebral Infarction: A Case Report. J Intern Med Taiwan. 2009; 20: 155-161.
10.Kumar S, Hammerschlag MR. Acute Respiratory Infection Due to Chlamydia pneumoniae: Current Status of Diagnostic Methods. Clinical Infectious Diseases. 2007; 44: 568-576.
11.Chamogeorgakis T, Lima B, Shafii AE, et al. Outcomes of axillary artery side graft cannulation for extracorporeal membrane oxygenation. The Journal of thoracic and cardiovascular surgery. 2013; 145: 1088-1092.
12.Cooper LT. Myocarditis. N Engl J Med. 360; 15: 1526-1538.
13.Odeh M, Oliven A. Chlamydial infections of the heart. Eur J Clin Microbiol Infect Dis. 1992; 11: 885-893.
14.Heinemann M, Kern WV, Bunjes D, et al. Severe Chlamydia pneumoniae Infection in Patients with Neutropenia: Case Reports and Literature Revie. Clinical Infectious Diseases. 2000; 31: 181-184.
15.Chlamydophila pneumoniae at the US National Library of Medicine Medical Subject Headings (MeSH).
16.Wang G, Burczynski F, Hasinoff B, et al. Infection of myocytes with chlamydiae. Microbiology. 2002; 148: 3955-3959.
17.Peeling RW. Laboratory diagnosis of Chlamydia pneumoniae infections. Can J Infect Dis. 1995; 6: 198-203.
18.Joshi R, Khandelwal B, Joshi D. Chlamydophila pneumoniae Infection and Cardiovascular Disease. N Am J Med Sci. 2013; 5: 169-181.