Acute Kidney Injury during Cirrhosis: A Prospective Study in Dakar
Author'(s): Alioune Badara FALL1*, Manssour MBENGUE2, Marie Louise BASSENE1, Marieme Polele FALL1, Salamata DIALLO1, Coumba CISSE1, Tene SIDIBE1, Alsine YAUCK1, Mouhamed SIDIBE1 and Nogaye NIANG1
1Cheikh Anta Diop University of Dakar, Hospital Aristide Le Dantec, Gastrology department, Dakar, Senegal.
2Cheikh Anta Diop University of Dakar, Hospital Aristide Le Dantec, Nephrology department, Dakar, Senegal.
*Correspondence:
Alioune Badara FALL, Cheikh Anta Diop University of Dakar, Hospital Aristide Le Dantec, Gastrology department, Dakar, Senegal.
Received: 10 Feb 2024 Accepted: 15 Mar 2024 Published: 22 Mar 2024
Citation: Alioune Badara F, Manssour M, Marie Louise B, et al. Acute Kidney Injury during Cirrhosis: A Prospective Study in Dakar. Gastroint Hepatol Dig Dis. 2024; 7(1): 1-7.
Abstract
Introduction: Patients with decompensated cirrhosis frequently develop acute kidney injury (AKI). It is observed in 40 to 80% of patients hospitalized for cirrhosis. It is often associated with poor prognosis. The objective of our study was to determine the epidemiological, clinical and evolutionary profile of AKI in cirrhotic patients, the etiological factors and predictors of mortality.
Method: We conducted a descriptive, analytical and multicenter study over a period of 18 months (January 2021 – July 2022). All cirrhotic patients hospitalized during the study period were included. AKI was diagnosed according to the criteria of the International Club of Ascites (ICA). The data was analyzed using SPSS version 23 software.
Results: We included 135 patients in our study. The prevalence of AKI was 52.60%. The average age of patients was 43.48 +/- 16 years, the majority of whom were men with a sex ratio of 2.46. Cirrhosis was secondary to the hepatitis B virus (HBV) in 60.70%. Ascites was present in 72.59% of patients, jaundice in 73.33%, upper gastrointestinal bleeding in 25.92% of patients. On biological screening, the average creatinine on D1 was 31.51 mg/l and 35.23 mg/l on D2. Stage 2 AKI was the most common stage found in our patients (32.39%). Functional renal failure (IRAF) was the most common cause of AKI (44.20%) followed by hepatorenal syndrome (HRS-AKI) found in 32.30% and acute tubular necrosis (ATN) found in 19.7%. The factors associated with the occurrence of AKI in multivariate analysis were sepsis and CHILD PUGH score (SPC). The overall mortality rate was 37.38% and the specific mortality of ARI was 28.89%. The factors associated with mortality in multivariate analysis were hepatocellular carcinoma, hepatic encephalopathy and progression of AKI.
Conclusion: The occurrence of an AKI during cirrhosis is a major turning point in the evolution of the disease with often poor prognosis. Early diagnosis is made using the ICA-AKI criteria. Its management must be fastacting and must, above all, resort to prevention of risk factors in the onset of AKI to improve prognosis.
Keywords
Introduction
Acute kidney injury (AKI) is a major complication whose appearance constitutes a major turning point in the evolution of cirrhosis. It affects 20 to 40% of hospitalized cirrhotic patients with often poor prognosis [1]. It is often triggered by precipitating events such as diuretic overdose, large volume paracentesis with lack of albumin supplementation, gastrointestinal bleeding and sepsis [2]. Many cases of AKI in patients with decompensated cirrhosis are functional in nature without structural lesions [3]. Most episodes of functional AKI (FAKI) respond to vascular filling. Unresponsive cases are considered as probable hepatorenal syndrome (HRS- AKI) or parenchymal AKI, which are often associated with significant morbidity and mortality [2-4]. Nearly 10% of patients with advanced decompensated cirrhosis present with SHR-AKI. This syndrome is mainly due to marked disturbances of the renal arterial microcirculation in cirrhotic patients [5]. MELD Scoring which takes account creatinine blood level in cirrhosis evolution appreciates the major prognostic influence of AKI in cirrhotic patients [6]. In Africa, few data are available on AKI during cirrhosis and its management remains a challenge due to the inaccessibility and unavailability of albumin and vasoactive drugs. This motivated our study, of which the objectives were to determine the clinical, epidemiological and evolutionary profile of AKI in cirrhotic patients, but also to determine the factors associated with its onset and predictors of mortality.
Patients and Method
The design of the research was a prospective, descriptive and analytical study conducted in the hepato-gastroenterology department of the Aristide Le Dantec hospital (HALD) and in the internal medicine department of Hôpital Principal of Dakar (HPD) during the period from January 1, 2021 to July 31, 2022. HALD and HPD are level 3 hospitals located in downtown Dakar. They offer various medical and surgical services, including gastroenterology, nephrology, internal medicine, ophthalmology, general surgery, digestive endoscopy and extra renal purification. Liver transplantation is not currently available in the study sites and in all of Senegal.
Inclusion Criteria
All adult patients over the age of 18 hospitalized for decompensated cirrhosis diagnosis Free and informed consent of all patients
Diagnosis of Cirrhosis
Clinical
The presence portal hypertension signs such as ascites, splenomegaly and collateral venous circulation or the presence of hepatocellular insufficiency signs such as jaundice, hepatic encephalopathy, spider angioma, gynecomastia, digital clubbing [7].
Biological
Impaired liver function with hypoalbuminemia, low prothrombin level, cytolysis, cholestasis characterized by hyperbilirubinemia and elevated alkaline phosphatase (ALP) [7].
CT scan signs of Cirrhosis
Dysmorphic liver, irregular contours, heterogeneous echostructure or signs of portal hypertension [8].
Diagnosis of AKI
Acute renal failure was diagnosed according to the criteria of the International Ascite Club (ICA) [9]:
- Increase in serum creatinine ≥ 3 mg/L (26.5 μmol /L) within 48 hours of admission.
- Serum creatinine > 5 times the basal serum creatinine measured in the previous 7 days.
The staging of AKI in this study was adopted from the ICA classification [9-10].
AKI stage 1 was defined as an increase in Creat ≥ 26.5 μmol /l or ≥1. 5 to 2 times from baseline, including ARF stages 1A (Creat ≥ 133 μmol /l) and 1B (Creat ≥ 133 μmol /l); AKI stage 2 was defined as an increase in Creat ≥ 2 to 3 times compared to baseline values; AKI stage 3 was defined as an increase in Creat ≥ 3 times over baseline or Creat ≥ 354 μmol /l. The initial stage of AKI was defined as the stage of AKI diagnosed for the first time during hospitalization on D1 [9-10]. AKI stage progression or lack of progression was determined by the last AKI stage close to discharge or death relative to the initial AKI stage. Hepatorenal syndrome (HRS- AKI) was diagnosed according to the new applicable criteria, however in our study filling was done more with isotonic saline than with albumin in most of our patients [11]. The Child- Pugh (CPS) prognostic score was used to assess the severity of liver disease during patient screening. Likewise, the MELD score (Model for end-stage liver disease) was calculated in all patients [12,13]. The following tests were performed: complete blood count, plasma glucose, liver function tests, kidney function tests, serum uric acid, serum calcium, serum phosphorus, prothrombin time/international normalized ratio (INR), hepatitis serology B and C, autoimmune hepatitis panel, ascites fluid analysis, chest X-ray and abdominal ultrasound. An abdominal CT scan, dosage of alkaline reserves and other examinations were carried out if necessary. Urine analysis (proteinuria, hematuria, leukocyturia) and urine electrolyte test were performed in all patients who presented with AKI.
Other settings
Spontaneous bacterial peritonitis (SBP) was defined by a PNN level in ascitic fluid greater than 250 elements/mm³ [14]. Sepsis was diagnosed according to SOFA criteria [15]. Acute tubular necrosis (ATN) was defined by an AKI that does not respond to vascular filling with a natriurese to kaliurese ratio greater than 1 and or a natriurese greater than 20 mmol/l [16]. Sudden-onset nephrotic syndrome (SNDB) was defined by ARI associated with nephrotic proteinuria (> 3g/24h) and hypoalbuminemia below 20g/l [17]. Acute nephritic syndrome (ANS) was defined by an ARF associated with proteinuria, hypertension and macroscopic or microscopic hematuria [18]. HCC was diagnosed by the presence of wash-in/wash-out enhancement pattern dynamic on CT scan.
Criteria for Non-Inclusion Patients with chronic renal failure Refusal to give consent Incomplete medical record
Ethical aspects
This study was conducted in accordance to the Declaration of Helsinki on Human Experimentation, Sixth Revision (October 2008), and approved by the Institutional Ethics and Review Board of HALD and HPD hospitals.
Data Analysis
Statistical Package for Social Sciences (IBM SPSS, version 23) statistical software was used to analyze the data. Descriptive statistics were performed for all variables and data were presented in appropriate tables. A subgroup analysis was performed to compare the sociodemographic characteristics and clinical characteristics of patients with AKI and patients without AKI. The etiology of liver cirrhosis and the prevalence of AKI were determined. Associations between acute renal failure and clinical or laboratory parameters were also determined using the Chi-square method. A multivariate regression analysis was performed to determine if any of the following clinical and laboratory parameters could be associated with acute renal failure or death.
Results
Characteristics of the study population
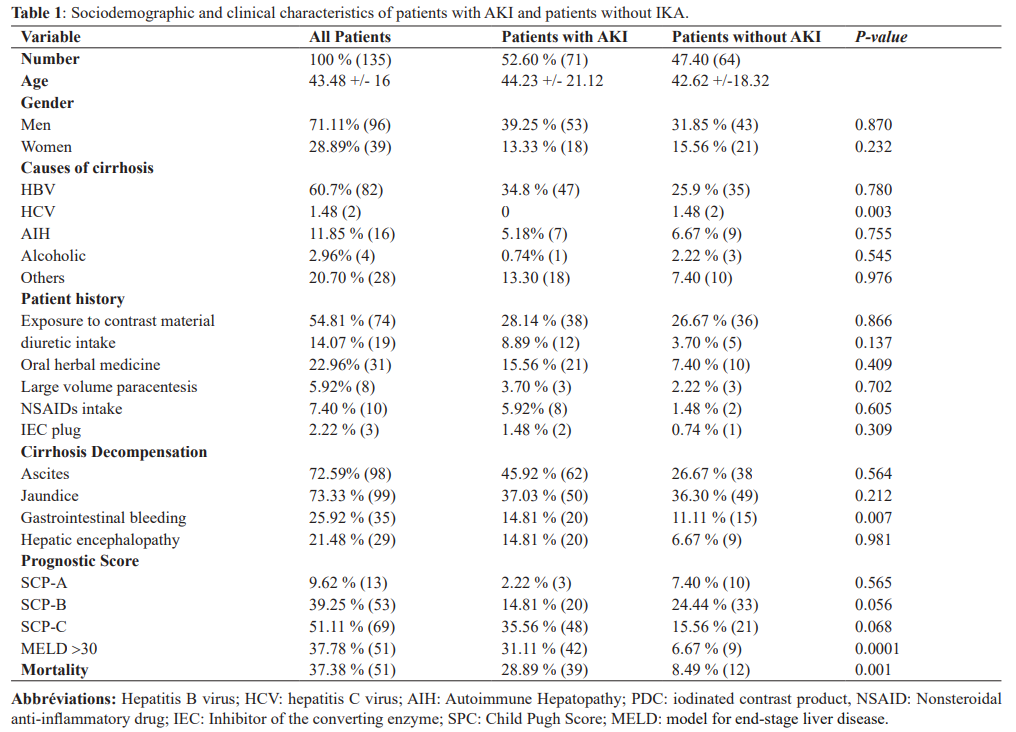
During the study period, 135 patients were included, they had an average age of 43 years, the majority of whom were men 71% (96) with a sex ratio of 2.46. The prevalence of AKI was 52.60% (or 71 patients). Cirrhosis viral B induced in 60.70% of cases, autoimmune in 11.85% of cases and indeterminate in 20% of patients, 46% of patients had a history of decompensation of cirrhosis in the ascites mode, a notion of recent exposure to iodine contrast product was noted in 50.3%, nonsteroidal anti- inflammatory drugs (NSAIDs) intake in 7, 40%, an ACE inhibitor intake in 5.18%, a large volume paracentesis in 3.51%, a diuretic intake in 14.07%. Concerning the prognosis 51.11% of the patients were classified CHILD C, 39.25% CHILD B and 9.62% CHILD A, the average MELD score was 23.3. Decompensation on the icteric mode was the most common reason for hospitalization (73.33%), followed by ascites 72.59% and hepatic encephalopathy in 21.4%. The decompensating factors were dominated by sepsis 28.9%, gastrointestinal bleeding in 25.9% and degeneration of cirrhosis into HCC in 32.5%.
Hepatic Biological Characteristics
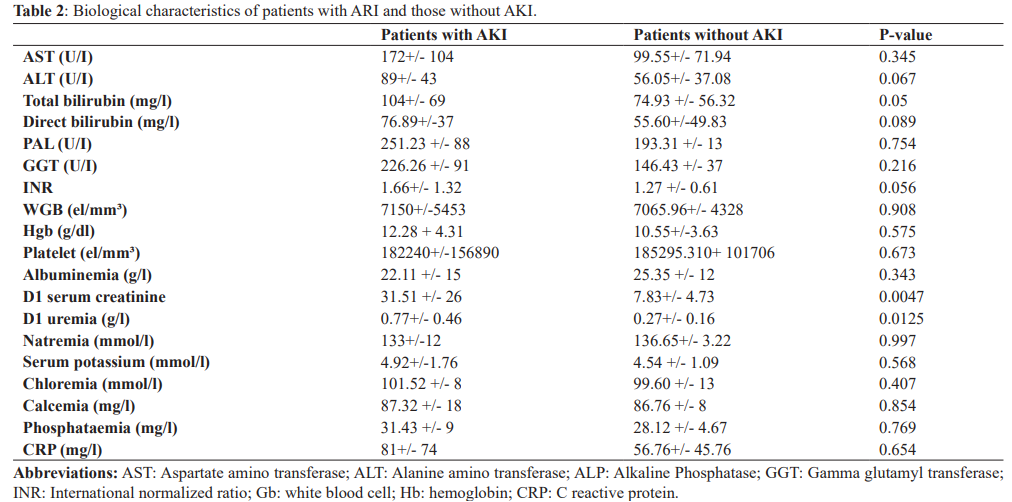
Cytolysis was noted in 79.2% with an average AST level of 73.6 IU/ l (+/- 21), hepatocellular insufficiency in 85.9% with an average TP of 50.1% (+/- 18 ,3) and an albuminemia of 23.6 g/l +/- 6.8.
Biological cholestasis was noted in 75.5% of patients with an average PAL of 222 U/I +/- 105 and an average total bilirubinemia of 47 mg/l +/- 38. The average hemoglobin level was 11, 4 g/dl +/- 4.34, mean platelet count 188.8 +/- 146, mean Gb level was 8980 +/- 7574. Mean protein level in ascitic fluid was of 17g/l +/- 12, a SBP was present in 23.07%.
Imaging
Liver was dysmorphic in 88.1% of patients, heterogeneous echostructure in 39.2%, signs of portal hypertension were present in 45.9% of patients. Esophageal varices were noted in 93.3% of patients. The diagnosis of hepatocellular carcinoma (HCC) was established in 26.7% of patients.
Characteristics of Kidney Involvement
Among the 135 hospitalized patients, 52.60% of patients had an AKI within 48 hours of admission. Mean serum creatinine on D1 was 31.51 mg/l +/- 26 and on D2 it was 35.23 mg/l +/- 20.09. The average glomerular filtration rate on admission was 45.50 ml/min according to EPI-CKD. The phosphor-calcic balance was normal in 81% of the patients, hyperkalemia was noted in 25.32% of the cases, hyponatremia in 32.35% of the patients, metabolic acidosis was objectified in 9.85%. Proteinuria was present in 14.07%, hematuria in 8.45 %, mean natriuresis was 26.82 mmol/24 +/-19.02 mmol/24 and mean kaliuresis was 48.47 mmol/l +/- 14.75. The AKI was stage 1 in 42.25% of cases, of which 16.90 were stage 1A and 25.35% stage 1B, stages 2 and 3 were respectively 32.39% and 25.35%. The AKI was of pre-renal origin in 42.25% of the cases, an HRS -AKI in 32.39% of the cases and a parenchymal AKI in 25.35% including 18.37% an ATN, an acute nephritic syndrome (ANS) in 4.22% and Sudden-onset nephrotic syndrome (SNDB) in 2.81%. A progression of the AKI was noted in 54.92%.
Etiological factors of AKI
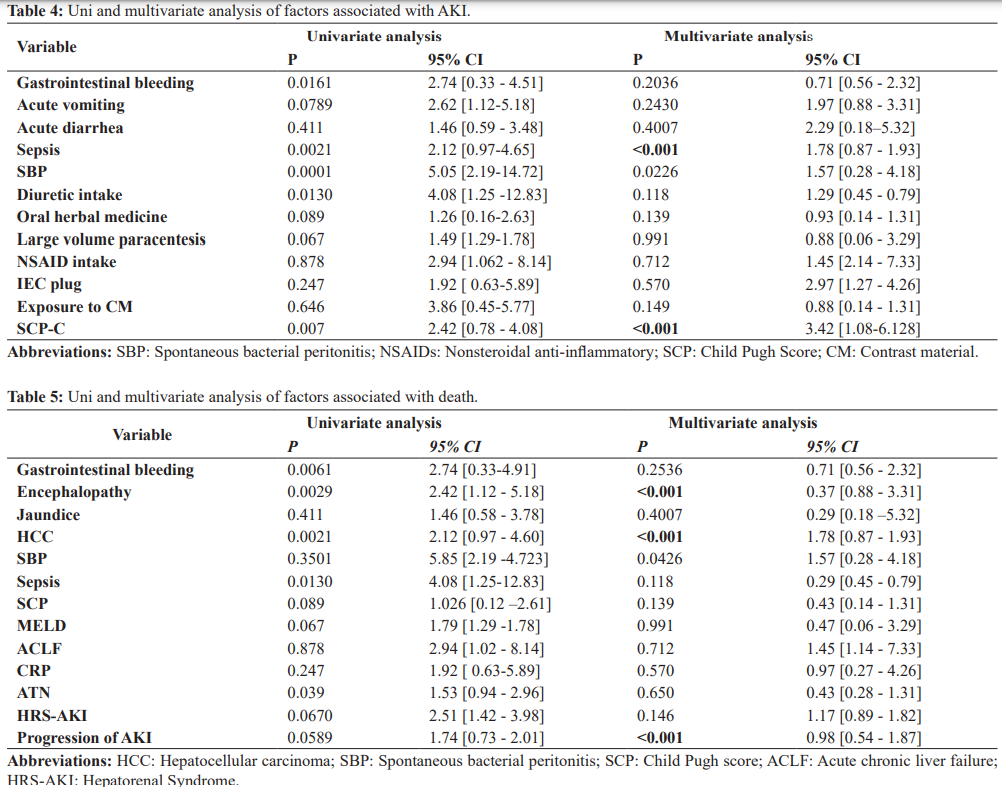
The etiological factors associated with AKI were sepsis, SBP, gastrointestinal bleeding, diuretic intake, CHILD C score. In multivariate analysis with logistic regression, only sepsis and CHILD C were associated with the occurrence of AKI.
Effect of AKI on survival
During the study period, the overall mortality rate was 37.38% (n=51) at 8 weeks. It was higher in patients with AKI (54.29%) than in patients without AKI (18.75%, P<0.001). In addition, patients with initial stage 1a (30%; P=0.001), stage 1b (38.89%; P<0.001), stage 2 (65.21%; P<0.001) and stage 3 (77.78%; P<0.001) had higher 8-week mortality rates higher than patients without AKI (18.75%; P<0.001). Regardless of the initial stage of AKI, patients with AKI progression had a higher mortality rate (87.18%) than patients who did not have progression (25.00%). In multivariate analysis HCC, AKI progression and hepatic encephalopathy were independent factors associated with death.
Discussion
In this present study we found a prevalence of 52.60% of AKI in cirrhotic patients according to the diagnostic criteria of the ICA-AKI. In Africa, some studies reported lower prevalence’s than ours. In Ivory Coast, Katia et al. reported a prevalence of 22.7% [20] and in Egypt Nahed et al. reported a prevalence of 28% [21]. This large difference in the prevalence of ARI is probably due to the diversity of the populations studied and the diagnostic criteria for ARI used. In 2015, the ICA proposed a new consensus definition of AKI and SHR-AKI in patients with cirrhosis which is essentially based on the dynamic of serum creatinine. A serum creatinine value >2.5mg/ dL has been deleted in the new consensus definition of AKI in cirrhotics. Higher prevalence’s were reported in America and China [22,23]. These high prevalence’s are probably linked to the use in these studies of more sensitive biomarkers than serum creatinine, such as plasma cystatin C and NGAL, which are markers not influenced by hepatocellular insufficiency and sarcopenia due to cirrhosis. FAKI is the leading cause of AKI in cirrhotics because it is favored by complications of portal hypertension (digestive hemorrhage, ascites), infections
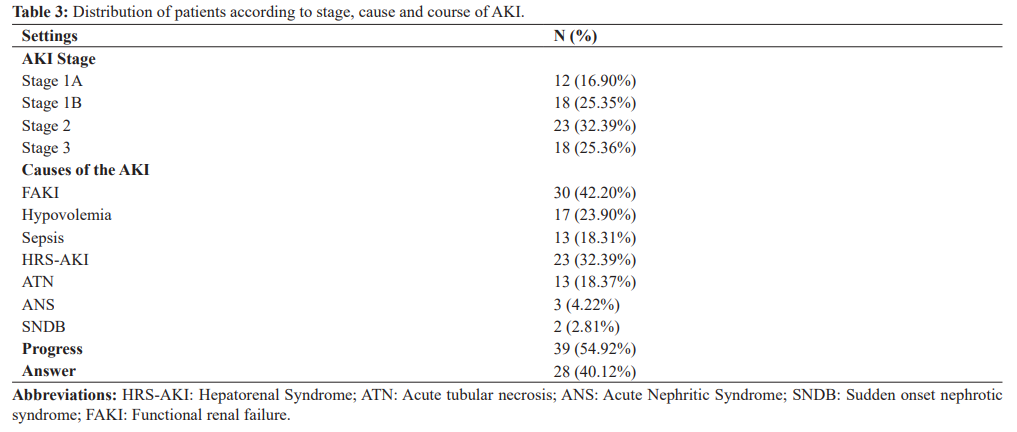
In our study, 42.2% of patients had an FAKI. Our data is consistent with literature [24-27]. In our research, the factors associated with the occurrence of AKI were diuretic intake, gastrointestinal bleeding, sepsis, SBP and CHILD-Pugh C score. These precipitating factors have already been reported in other studies; Gomes et al. [28] in their study reported infection and hypovolemia as the main precipitating factors for AKI, Belcher et al. have also published the latter as the main precipitating factors of AKI in cirrhotic patients. Diuretic intake and gastrointestinal bleeding are reported in several studies as frequent etiologies of FAKI in cirrhotic patients [25,29]. Indeed the use of high doses of diuretics in decompensated cirrhosis exposes to a risk of AKI due to the dehydration that they can induce on a state of pre-existing relative hypovolemia, favored by the circulatory hyperkinesia state, secondary to portal hypertension. Gastrointestinal bleeding is also a significant risk factor for the occurrence of AKI during cirrhosis, mainly due to the worsening of relative hypovolemia but, above all, the risk of infection secondary to intestinal bacterial proliferation. The estimated mortality of cirrhotic patients with ARI is about 50% in one month.
In our study, mortality at 8 weeks was 54.29%. Western and Asian studies have reported lower prevalence’s than ours. In America, the Brazilian study by Gomes et al. reported a mortality rate of 45% [28] and the American study by Belcher et al., a rate of 46% [30]. In Europe Fagundes et al. reported a rate of 25% [26]. In Asia, the Taiwanese study by Yun et al. reported a mortality rate of 23% [31] and Arora in India a mortality rate of 34% [32]. In Africa a Ghanaian study reported a mortality of 64%, about similar to ours. Variations in mortality rates may be due to differences in the underlying factors of AKI, etiology and severity of liver disease, stage of AKI, and resources available for managing patients. The high mortality rate in our regions may be due to the diagnostic delay of cirrhosis which is often diagnosed in decompensated phase, unavailability and inaccessibility of vasoactive drugs and albumin which improve the prognosis of AKI in cirrhotic patients. The prognosis of AKI in cirrhosis is also influenced by other complications of cirrhosis such as hepatic encephalopathy, HCC and gastrointestinal bleeding. In our present study, HCC and hepatic encephalopathy were correlated with mortality in multivariate analysis, Uday et al. and Lins et al. reported similar results [33]. Progression of renal failure should be considered as an independent risk factor associated with mortality in cirrhosis. Therefore, patients with progression should receive more aggressive treatment to optimize effective arterial blood volume and reduce the risk of progression to HRS-AKI or NTA [34].
Conclusion
AKI is a frequent and impressive complication in cirrhotic patients. It is often favored by factors such as sepsis, gastrointestinal bleeding, diuretic intake but also the advance stage of the disease. Its prognosis is poor because of its complications and cirrhotic related complications, thus the requirement for early and appropriate care from diagnosis of AKI.
References
- Garcia –Tsao G, Parikh CR, Viola A, “Acutekidneyinjury in cirrhosis” Hepatology. 2008; 48: 2064-2077.
- Salerno F, Gerbes A, Ginès P, et Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007; 56: 1310-1318.
- Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008; 48: 2064-2077.
- De Carvalho JR, Villela –Nogueira CA, Luiz RR, et “Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites,” Journal of Clinical Gastroenterology. 2012; 46: e21-e26.
- Bacon RB. Cirrhosis and its complications. In: Kasper DL, Fauci AS, Hauser SL, Congo DL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 19th ed. New York (NY): McGraw Hill; 2015. 2058-2067.
- Fede G, Tsochatzis E, Germani G, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol. 2012; 56: 810-818.
- Smith A, Baumgartner K, Bositis Cirrhosis: Diagnosis and Management. Am Fam Physician. 2019; 100: 759-770.
- Soresi M, Giannitrapani L, Cervello M, et al. Noninvasive tools for the diagnosis of liver World J Gastroenterol. 2014; 20: 18131-18150.
- Angeli P, Gines P, Wong F, et Diagnosis and management of lesions renal in patients with cirrhosis:revised consensus recommendations from the International Ascites Club. Intestine. 2015; 64: 531-537.
- Rosi S, Piano S, Frigo AC, et al. New ICA criteria for the diagnosis of acute kidney injury in cirrhotic patients: can we use an imputed value of serum creatinine? Liver 2015; 35: 2108-2114.
- Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. 2020; 370: 26-87.
- Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008; 28: 110-122.
- DellaVolpe J, Al- Khafaji A. Lesions renal acute before and after liver transplantation. J Intensive Care Med. 2019; 34: 687-695.
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010; 53: 397-417.
- Strnad P, Tacke F, Koch A, et al. Liver - guardian, modifier and target of Nat Rev Gastroenterol Hepatol. 2017; 14: 55-66.
- Schrier RW, Shchekochikhin D, Ginès P. Renal failure in cirrhosis: prerenal azotemia, hepatorenal syndrome and acute tubular Nephrol Dial Transplant. 2012; 27: 2625-2628.
- Endo Y, Matsushita H, Nozawa Y, et al. Glomerulonephritis associated with liver cirrhosis. Acta Pathol Jpn. 1983; 33: 333-346.
- Fukuda Y. Renal glomerular changes associated with liver cirrhosis. Acta Pathol Jpn. 1982; 32: 561-574.
- Trinchet JCHepatocellular carcinoma in 2014: Current situation and future prospects. Interventional Image. 2014; 95: 705-708.
- Ttia KA, N'dri Yoman AT, Mahassadi AK, et al. Impact of renal failure on survival of African patients with cirrhosis. J Kidney Dis Transpl. 2008; 19: 587-592.
- Nahed K, Morsy KHRenal failure after upper gastrointestinal bleeding in cirrhotic patients in Upper Egypt. Arabic J Gastroenterol. 2012; 13: 139-144.
- Maiwall R, Pasupuleti SSR, Bihari C, et al. Incidence, risk factors, and transition outcomes of kidney injury Acute to chronic kidney disease in cirrhosis: a prospective cohort study. Hepatolo. 2020; 71: 1009-1022.
- Xiong J, Pu L, Xiong H, et al. Evaluation of the criteria of hepatorenal syndrome type of acute kidney injury in patients with cirrhosis admitted to ICU. Scand J Gastroenterol. 2018; 53: 1590-1596.
- Pukar T, Kc S, Hamal AB, et al. Prevalence of Acute Kidney Injury in Patients with Liver Cirrhosis. JNMA J Nepal Med Assoc. 2020; 58: 554-559.
- Hueon K, Im CB, Lee SS, et Impact of acute kidney injury on mortality in patients with acute variceal bleeding. BMC Gastroenterol. 2021; 21: 290.
- Fagundes C, Barreto R, Guevara M, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in J Hepatol. 2013; 59: 474-481.
- Garcia Garcia A, Tellez L, Rodriguez- Gandia MA, et al. Incidence, predictive factors and impacts of acute kidney injury in cirrhotic patients hospitalized for Liver Int. 2018; 38: 285-294.
- Gomes CGO, de Andrade MVM, Resende Guedes L, et al. Clinical Aspects and Prognosis Evaluation of Cirrhotic Patients Hospitalized with Acute Kidney Injury. Can J Gastroenterol Hepatol. 2019; 2019: 6567.
- Fonseca Vaz, da Cunha VNR, Cunha-Silva M, et Evolution of diagnostic criteria for lesions renal injuries in patients with decompensated cirrhosis: a prospective study in a tertiary teaching hospital.Clin Res Hepatol Gastroenterol. 2020; 44: 551-563.
- Belcher AS, Parada XV, Endres P, et al. HRS -HARMONY study investigators. Urinary NGAL as a Diagnostic and Prognostic Marker for Acute Kidney Injury in Cirrhosis: A Prospective Study. Wink Transl Gastroenterol. 2021; 12:
- Hsieh YC, Lee KC, Chen PH, et al. Acute kidney injury predicts mortality in cirrhotic patients with gastric variceal bleeding. J Gastroenterol Hepatol. 2017; 32: 1859-1866.
- Arora MS, Kaushik R, Ahmad S, et al. Profile of Acute Kidney Injury in Patients with Decompensated Cirrhosis at a Tertiary-Care Center in Uttarakhand, India. Dig Dis.2020; 38: 335-343.
- Lins PR, Carvalho Padilha WS, Magalhaes Giradin Pimentel
- Risk factors, mortality and acute kidney injury outcomes in cirrhotic patients in the emergency department. BMC Nephrol. 2018; 19: 277.
- Wong F, Reddy KR, O'Leary JG, et al. Impact of Chronic Kidney Disease on Outcomes in Cirrhosis. Liver Transpl. 2019; 25: 870-880.