Age and Performance Status Significantly Influence Outcome despite the Advent of New Therapy Options in Multiple Myeloma
Author'(s): Geothy Chakupurakal1*, Stefan Feiten2, Roland Schnell3, Tilman H Steinmetz4, Lothar Müller5, Oswald Burkhard6, Ute Braun7, Peter Ehscheidt8, Ingo Tamm9, Bernhard Rendenbach10 and Rudolf Weide1
1Praxis fuer Haematologie und Onkologie, Koblenz, Germany
2Institut fuer Versorgungsforschung in der Onkologie, Koblenz,Germany.
3pioh - Praxis Internistische Onkologie und Haematologie, Frechen/ Cologne, Germany.
4MV-Zentrum fuer Onkologie und Haematologie, Cologne,Germany.
5Onkologie UnterEms, Leer, Germany.
6Internistische Gemeinschaftspraxis Haematologie, Onkologie,Palliativmedizin, Worms, Germany
7Onkologische Schwerpunktpraxis Braun und Huenermund,Ludwigshafen, Germany.
8MVZ Haematologie/Onkologie, Neuwied, Germany.
9Onkologische Schwerpunktpraxis Kurfuerstendamm, Berlin,Germany
10Onkologisch/nephrologische Schwerpunktpraxis, Trier, Germany.
*Correspondence:
Geothy Chakupurakal, Praxis fuer Haematologie und Onkologie, Neversstr 5, 56068 Koblenz, Germany, Tel: +49 261 304930.
Received: 25 Feb 2024; Accepted: 30 Mar 2024; Published: 07 Apr 2024
Citation: Chakupurakal G, Feiten S, Schnell R, et al. Age and Performance Status Significantly Influence Outcome despite the Advent of New Therapy Options in Multiple Myeloma. Cancer Sci Res. 2024; 7(2): 1-6.
Keywords
Introduction
Multiple Myeloma (MM) is the second most common haematological malignancy in the western world and is a disease predominantly of the elderly with a median age, at the time of diagnosis, of 65 [1-3]. In this study the treatment and outcome of MM patients managed in 10 outpatient practices in Germany over a period of 6 years was analysed. Our aim was to understand the course of the disease in an unselected patient group, to evaluate the role of different available therapeutic options and study the challenges faced in the management of this patient cohort.
Materials and Methods
We retrospectively reviewed all consecutive patients with a confirmed diagnosis of MM irrespective of their treatment requirement between 01/2012–12/2018. Patients with monoclonal gammopathy of undetermined significance (MGUS), solitary plasmacytoma and plasma cell leukaemia were excluded. Electronic files were searched for relevant codes of the international classification of diseases to identify patients. The extracted data was anonymised and imported into a database and analysed statistically using SPSS 19.
This study was approved by the ethics committee of Rhineland- Palatinate, Germany, approval number 837.472.16 (10791). An institutional review board approval was not necessary.
Results Patient Demographics
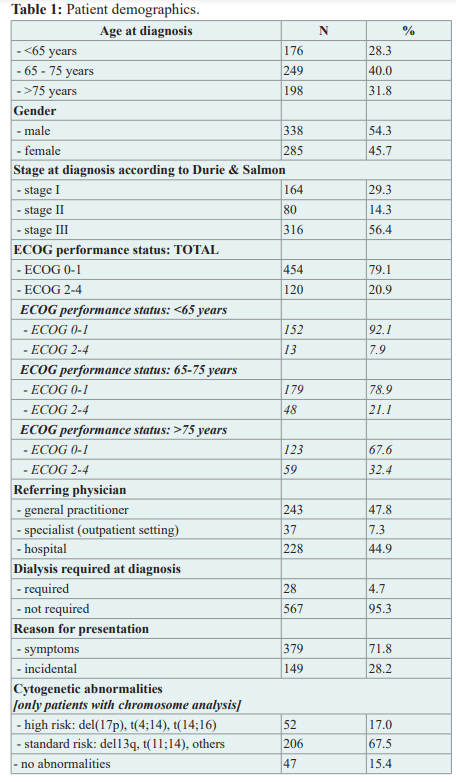
A total of 623 patients with MM were identified. The median age at diagnosis was 71 (range 25-92) and more than half (54%) were male. 176 patients (28%) were <65 years old, 249 (40%) 65-75 years and 198 (32%) >75 years. Eastern Cooperative Oncology Group Performance Status (ECOG) was available on 574 patients. 454/574 (79%) had a good ECOG (0/1). 120/574 (21%) had a score of ≥2. Only 67% (302/454) of the patients with a good ECOG were<65. Almost 90% (107/120) of the patients with a poor ECOG (≥2) were ≥65. Table 1.
Diagnosis
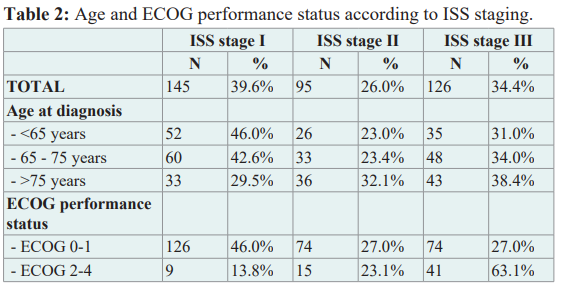
72% of the patients were symptomatic at the time of first presentation. 17% had renal insufficiency at the time of diagnosis and 5% required dialysis. The majority received a bone marrow biopsy (above 90%) and cytogenetic analysis was performed in approximately 50%. Cytogenetic abnormalities identified standard risk in 83% [del(13q), t(11;14), other and / or no abnormalities] and high risk in 17% [del(17p), t(4;14), t(14;16)]. At the time of first presentation 56%, 14% and 29% of patients were in stage III, stage II and stage I according to Durie & Salmon [4,5]. ISS staging [6] based on age and ECOG is shown in table 2. Staging based on ISS at diagnosis was not available in 257/623 patients (41%), the revised ISS was not available at all due to lack of data.
Treatment
One fifth of the patients (105/623; 17%) received no treatment. 518/623 patients (83%) received at least one line of treatment of whom 159 (30%), 208 (40%) and 151 (30%) were <65, 65-
75 and >75 years respectively. Bortezomib/cyclophosphamide/ dexamethasone (VCD) and bortezomib/melphalan/prednisone (VMP) were the commonest first line therapies given to 30% and 21% respectively. 343/518 (66%) patients did not receive an autologous stem cell transplantation (auto-SCT). Lenalidomide/ dexamethasone (RD) was the commonest (40%) second line treatment of choice for those who did not receive an auto-SCT. On average patients received a median of 2 therapy lines (range 1-11). Age, <65, 65-75 and >75 years, made no difference to the number of therapy lines i.e. 2 (1-11), 2 (1-8) and 2 (1-8) respectively. An allogeneic stem cell transplantation (allo-SCT) was given to 4 patients as 3rd (n=2), fifth or eighth therapy option. Newer agents like daratumumab, elotuzumab, carfilzomib and pomalidomide were administered after three or more lines of treatment. An overview of the first four therapy lines for patients who had no auto-SCT is shown in the supplementary figure 1a.
After the initial induction and consolidation 175/518 (34%) received an auto-SCT. The median age of the patients, who received an auto-SCT was 60 (range 35-79). Induction and consolidation regimens with the auto-SCT was considered as one therapy line. Bortezomib/cyclophosphamide/dexamethasone (VCD) in 47%, followed by bortezomib/doxorubicin/dexamethasone (PAD) in 18% were the commonly used inducing regimens prior to an auto-SCT. Supplementary figure 1b. 23/175 (13%) of these patients received a tandem transplantation. After auto-SCT 78/175 (45%) received a maintenance therapy with lenalidomide, 97/175 (55%) had no lenalidomide maintenance. 77% had lenalidomide maintenance following bortezomib-containing induction regimens, mainly VCD, PAD and VD. 2nd, 3rd and 4th line treatments following induction +/- lenalidomide maintenance are depicted in the supplementary figure 1b.
Hospital Admissions
52% (326/623) were hospitalised after their initial diagnosis, with a median of 3 hospitalisations (range 1-17). The median cumulative number of hospitalised days was 31 (range 2-361). 56% of all hospital admissions were unplanned or as an emergency (comorbidities, fractures, renal failure, pain, toxicities, infections, deterioration of general condition). In 34%, hospitalizations were elective (high-dose therapies with auto-SCT, allo-SCT, other inpatient myeloma therapy) and in 10% the reason could not be determined. 40%, 45% and 54% of the patients aged <65, 65-75 and >75 respectively, required emergency hospital admissions.
Supportive Therapy and Toxicities
39% required no analgesia whereas 27%, 11% and 23% received non opioids, weak opioids or strong opioids respectively. 4% required a palliative radiotherapy as pain management. Vertebroplasty or kyphoplasty was offered to 8% of the patients. 60% of the patients received a bisphosphonate treatment. Zoledronic acid and ibandronic acid were the commonly used bisphosphonates in 64% and 28% of the patients respectively. 3% of patients developed an osteonecrosis over the course of the observation period based on the documented data. Blood or platelet transfusions were offered to 41% and 9% of the patients. Patients received a median of 4 blood (range 1-50) and 2 platelet (range 1-29) transfusions. 10% were administered immunoglobulins and the most important cause for immunoglobulin substitution was recurrent infections. 11% were given erythropoietin. 32% (201/623) of the patients died during the observation period of median 26 months (range 0-92). 49% (98/201) died due to MM, 1% (3/201) due to treatment toxicities and 16% (33/201) due to their comorbidities. Amongst these patients 17%, 33% and 45% were <65, 65-75 and >75 years respectively. In 33% (67/201) the cause of death was not available. 48% (96/201) died in hospital. A small proportion of patients died at home (27/201, 13%) or a hospice (13/201, 6%).
Overall Survival (OS)
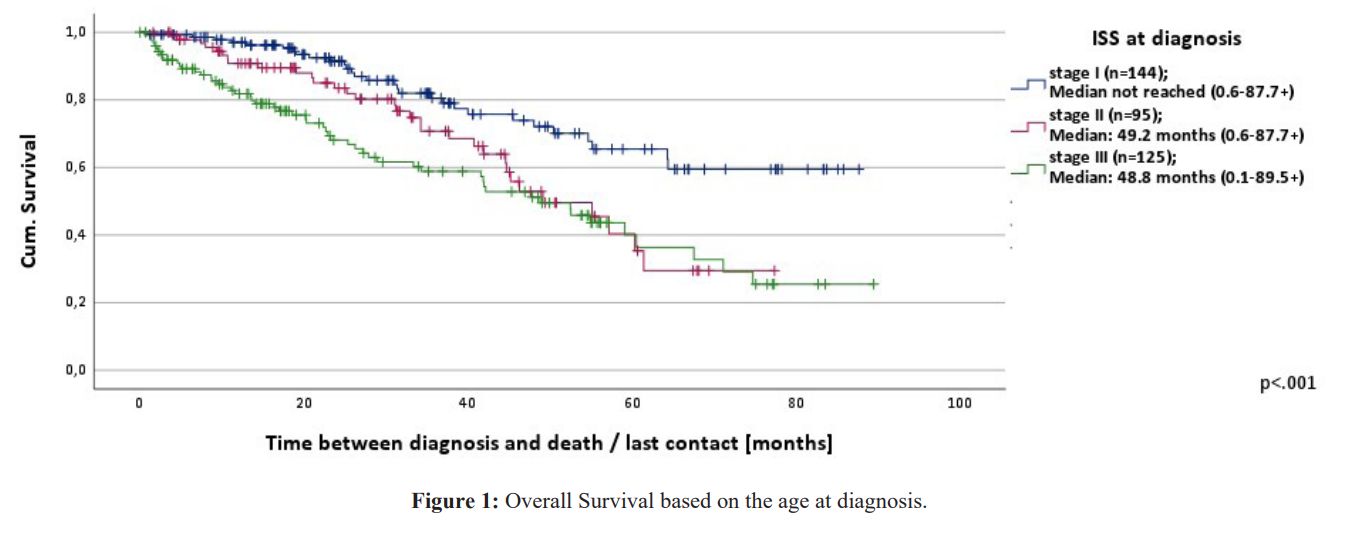
The median OS from the time of diagnosis for the whole cohort (n=619) was 64.4 months (range 0.1-92.2+) and the progression free survival (PFS) 25.0 months (range 0.6-92.2+) with a 5-year OS of 54%. Data on 4 patients who received treatment was not available. OS according to the three age cohorts is depicted in figure.
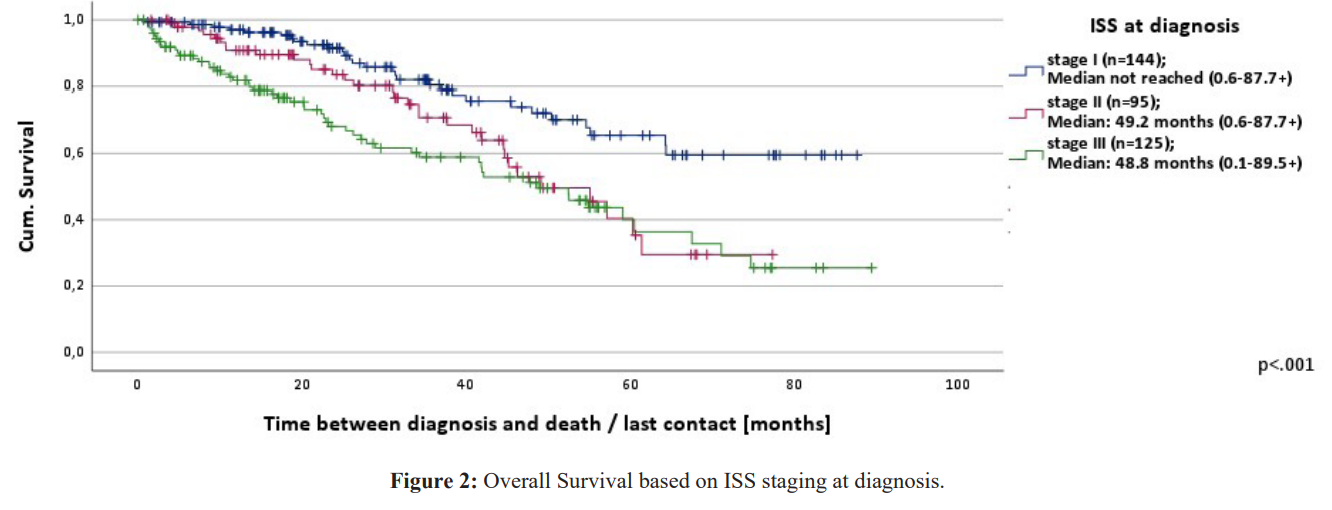
- OS from the time of first treatment (n=499) was on average 56.3 months (range 0.2-89.3+) and PFS 19.8 months (range 0.0-88.8). The OS of the patients from the time of treatment based on age was for the age groups <65, 65-75, >75 median not reached (range 0.3- 84.0+), 56.3 months (range 0.2-89.3+) and 40.6 months (range 0.2- 79.1+) respectively. The differences were statistically significant p< 0.001. Patients with a good ECOG survived 64.1 months (range 0.7-89.3+) and those with a score of ≥2 survived 21.4 months (range 0.2-61.6+). This was also statistically significant p< 0.001.OS from the time of diagnosis according to ISS stage groups is depicted in figure 2. The differences in OS between the patient groups from the time of first treatment based on the ISS stage I, II and III were 61.4 (range 2.0-82.9+), 58.7 (range 4.2-81.0+) and 54.6 months (range 0.2-89.3+) respectively. These differences were not statistically significant (p=0.384). Patients who received an auto-SCT (n=173) had a significantly longer OS (median not reached, range 8.0-91.4+) than those who were not eligible for an auto-SCT (n=340) (OS 47.9 months, range 0.6-92.2+) (p< 0.001). Supplementary figure 2a. Median was not reached in OS of patients who received a maintenance treatment with lenalidomide after auto-SCT and in those who didn't. Supplementary figure 2b. The median OS for patients who received an auto-SCT was not reached in stages I-III [7-15].
Conclusions
The advent of new therapy options has made MM a chronic disease with a better prognosis despite the aging population affected by it. Approximately every third patient (32%) in this study was >75 and around 20% had an ECOG > 2. This reflects our aging society and a significant proportion of these patients cannot be treated according to guidelines due to their frailty, comorbidities or other factors. 70% of patients in our study could be included in the earlier International Staging System (ISS) stage I and II comparable to Steinmetz et al. [16]. Studies comparing the experiences of physicians in different European countries, Switzerland and the UK showed comparable parameters and outcomes with respect to presenting symptoms and distribution across stages [15,17]. The OS of this patient group at 5 and 10 years in Germany is 50% and 30% respectively [4]. OS is affected by a variety of factors such as patient characteristics, tumour stage, cytogenetic abnormalities and response to therapy [13]. 76% of the patients above 75 received at least one line of treatment. In our study patients who were >70 years at diagnosis had a 5-year OS of 41% compared to 67% in patients < 70.53% of the patients received a bortezomib containing regimen and physicians preferred lenalidomide containing regimens as the second line in the study by Yong et al. comparable to our data [15]. In our cohort around a fifth (17%, 105/623) of the patients received no treatment in contrast to other studies probably due to the fact that we included patients based on diagnosis and not treatment [15-17]. 34% (175/518) of the patients who received an auto-SCT had a superior OS (median not reached) than those who were not eligible for this treatment (47.9 months). The advantages of auto-SCT and lenalidomide maintenance are comparable to similar studies analysing real world data [18-21]. Patients >75 who received treatment had a median OS of 43 months in our study.
The current staging systems give a better representation of different subgroups due to the addition of LDH and chromosomal abnormalities [6]. Unfortunately, due to lack of data this analysis was not possible. The depth of response after each line of treatment and exact treatment free intervals could not be extracted. We were unable to ascertain the duration of treatment for each therapy line. Data on treatment related toxicities and grades were not available. Pain was a significant problem for the patients and 60% received bisphosphonate treatment comparable to data from Yong et al. [15]. The role for palliative care in patients with MM is highlighted in the paper by Palotti et al. [22]. Another cross- sectional study across different European countries showed that at the time of death only 37% patients were undergoing treatment and 51% were receiving supportive care [20]. Only a small proportion of the patients (20%) died at home or in a hospice. Early inclusion of the palliative services in the care of the patient and the incorporation of the family or care-givers in the ongoing treatments will help in improving the end of life care for these patients. The treatment options and prognosis of patients with MM have improved tremendously in the last decade. Treatment of these patients can be delivered safely and in concordance with the current standards in the outpatient setting. Our study highlights the influence of age and performance status on outcome though these parameters are not included in the ISS or R-ISS. We aim to conduct future studies to update the information gathered in this study. A comprehensive multidisciplinary network is vital in facilitating this care from diagnosis to the end of patient care. Future clinical studies need to address the problems with an aging population with multiple comorbidities. Such studies will aid the decision-making with regards to treatment options available for this patient cohort without reducing their quality of life.
Declarations Acknowledgement
We would like to thank all MM patients and the medical documenters who transferred the data from the patient files.
Funding Sources
This work was supported through a restricted grant from Celgene GmbH, Munich, Germany, medac GmbH, Wedel, Germany and Octapharma GmbH, Langenfeld, Germany to the Institute for Health Services Research in Oncology (Institut fuer Versorgungsforschung in der Onkologie), Koblenz, Germany. Celgene, medac and Octapharma were not involved in gathering, analysing, or interpreting the data or in the writing of the manuscript. The views expressed are those of the authors and not necessarily those of the funders.
Informed Consent and Ethics
Only anonymized routine data was collected, which is why patient consent was not required. A positive vote from the Rhineland- Palatinate Ethics Committee was available (Approval number 837.472.16 (10791).
References
- Rajkumar SV, Dimopoulos MA, Palumbo A, et International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15: e538-548.
- Krebs in Deutschland für 2015/2016. Ausgabe. Robert Koch-Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). Berlin, 2019.
- Kyle RA, Gertz MA, Witzig TE, et Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78: 21-33.
- Cancer in Germany 2017/2018. 13th edition. Robert Koch Institute (ed.) and the Association of Population-based Cancer Registries in Germany (ed.). Berlin, 2022: 138-1341.
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011; 364: 1046-1060.
- Palumbo A, Avet-Loiseau H, Oliva S, et Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015; 33: 2863-2869.
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple Intergroupe Français du Myélome. N Engl J Med. 1996; 335: 91-97.
- Giralt S, Stadtmauer EA, Harousseau JL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia. 2009; 23: 1904-1912.
- Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell Leukemia. 2009; 23: 1716-1730.
- Anderson KC, Kyle RA, Rajkumar SV, et Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008; 22: 231-239.
- Goldschmidt H, Lokhorst HM, Mai EK, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 Leukemia. 2018; 32: 383-390.
- Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 Lancet (London, England). 2017; 389: 519-527.
- Kumar SK, Lee JH, Lahuerta JJ, et Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012; 26: 149-157.
- Kastritis E, Terpos E, Roussou M, et al. Evaluation of the Revised International Staging System in an independent cohort of unselected patients with multiple Haematologica. 2017; 102: 593-599.
- Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world Br J Haematol. 2016; 175: 252-264.
- Steinmetz T, Ernst A, Hellmich M, et al. Effectiveness of Long-Term Treatment of Multiple Myeloma in Regular Care: Comparison of a Longitudinal and a Cross-Sectional Analysis Oncology research and treatment. 2021; 44: 662- 671.
- Raab MS, Cavo M, Delforge M, et al. Multiple myeloma: practice patterns across Europe. Br J Haematol. 2016; 175: 66-76.
- Mair M, Straka C, Buratti T, et al. Clinical outcomes and prognostic factors in patients with multiple myeloma in South Tyrol: a retrospective single-center analysis. Ann Hematol. 2020; 99: 1031-1040.
- Greipp PR, San Miguel J, Durie BG, et International staging system for multiple myeloma. J Clin Oncol. 2005; 23: 3412-3420.
- Mohty M, Cavo M, Fink L, et al. Understanding mortality in multiple myeloma: Findings of a European retrospective chart Eur J Haematol. 2019; 103:107-115.
- Ruggiero SL, Dodson TB, Aghaloo T, et American Association of Oral and Maxillofacial Surgeons' Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2022; 80: 920-943.
- Pallotti MC, Rossi R, Scarpi E, et al. Patients with multiple myeloma referred for palliative care consultation: from retrospective analysis to future directions to improve clinical Support Care Cancer. 2022; 30: 2293-2298.