Antibiotic Susceptibility Profile of Salmonella spp and Shigella spp Isolated from Commercial Frozen Chicken Sold in three Markets within Awka Metropolis
Author'(s): Muna F. Okoli, Malachy C. Ugwu*, Kene C.Ezejiegu, Ugonna Morikwe
Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka Nigeria.
*Correspondence:
Malachy C. Ugwu, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka Nigeria; E-mail: mc.ugwu@unizik. edu.ng.
Received: 30 July 2021; Accepted: 25 August 2021
Citation: Okoli MF, Ugwu MC, Ezejiegu KC, et al. Antibiotic Susceptibility Profile of Salmonella spp and Shigella spp Isolated from Commercial Frozen Chicken Sold in three Markets within Awka Metropolis. Clin Immunol Res. 2021; 5(2): 1-4.
Abstract
Background: Contamination of chicken meat by bacteria contributes to the increasing incidence of food –borne infections in humans. This study was aimed at providing data on the antibiotic susceptibility profile of Salmonella and Shigella species from frozen chicken sold within the Awka metropolis.
Methodology: A total of 100 frozen chicken samples were obtained from different markets within the Awka metropolis. Smear samples were gotten from each chicken sample using sterile swab stick. Salmonella and Shigella samples were isolated after culturing the samples with Salmonella –Shigella agar and pure isolates identified morphologically, by Gram and biochemical characteristics. Antibiotic susceptibility test was performed using the disc diffusion method as recommended by CSLI. Isolates that showed resistance to any of the third-generation cephalosporin used were further subjected to the Double Disk Synergy Tests (DDST).
Results: Of the 100 samples, 25 Shigella spp and 51 isolates of Salmonella were isolated. All Salmonella and Shigella isolates were resistant to the β-latam antibiotics. A 100% of the shigella isolates were resistant to all generation cephalospoin used. The bacterial spps were however, sensitive to nitrofurantoin and ciprofloxacin.
Conclusion: This study has shown that frozen chicken serves as sources/ reservoir for bacterial infections. The isolates were resistant to the cephalosporins but sensitive to ciprofloxacin and nitrofurantoin.
Keywords
Introduction
Salmonellosis is a disease condition caused by Salmonella spp globally affecting both humans and animals. Although the condition of the disease in humans normally presents with a case of mild gastroenteritis, life-threatening systemic infections are common, especially among high-risk categories. Sources and modes of transmission of non-typhoidal Salmonella are still poorly understood in Africa due to the lack of coordinated national epidemiological surveillance systems and poor healthcare system. Salmonella is one of the leading causes of infection in food producing animals especially poultry and this has a direct impact on the global marketing of the respective food-producing animals as well as animal-derived food products [1].
Shigellosis is a disease of public health concern in terms of its socio-economic impacts. Shigella infection in humans is projected to be responsible for at least 80 million cases of bloody diarrhea and annually and are detected in the stools of 5%–18% of patients with travelers’ diarrhea [2-5]. About 99% of the infections occur in developing countries. Shigellosis occurs mainly in resource- poor, crowded communities lacking adequate sanitation, safe water and good living conditions. It is spread by direct contact with an infected host or through fecal-oral transmission by eating contaminated food and water [6,7].
Salmonella has been detected in retail foods e.g chicken. Retail chicken is the most common carrier of Salmonella [8]. Application of potentially contaminated water during processing as well as the quality of food packaging materials could predispose the foods to pathogenic microorganisms [9].
Emergence and rapid spread of antimicrobial resistance among microbial population has become a global health challenge. Some of these pathogens isolated from the environment have shown an increased resistance to antbiotics since they have developed a number of elaborate mechanisms for acquiring and diseminating genetic determinants [6]. The need to know the data in resistance bacteria’s profile is geared towards better understanding of the distribution and antibiotic susceptibility in retail and frozen foods.
Numerous data are available on the incidence of shigelloiosios from live chicken [1] and from animal feed [10]. However, little or no data exists on the incidence of shigellosis and enteric typhoid fever infection emanating from frozen chicken samples. This study is aimed at providing data on the antibiotic susceptibility profile of Salmonella and Shigella species from frozen chicken sold within the Awka metropolis.
Materials and Methods
Culture media and reagents
Nutrient Broth, Mueller-Hinton Agar (Oxoid Limited, England), Salmonella Shigella agar, Nutrient agar, Oxidase reagent, hydrogen peroxide, kovac’s reagent, peptone water, lugols iodine, safranin, immersion oil, absolute ethanol (sigma-aldrich, Germany).
Equipment’s
Equitron Partially Automatic Autoclave (Medica Instrument Manufacturing CO., India), Hot Air Oven (Genlab, UK), Incubator (Genlab, UK), Electronic weighing balance (Ohaus Corp., USA).
Study deign, sample collection
One hundred (100) frozen chicken samples were collected from three (3) markets within the Awka metropolis. Smear samples were obtained from each chicken sample, with the aid of a sterile swab stick. The samples were grown in a nutrient agar for 24 hours at 37°C ± 1°C after which Salmonella and Shigella species were isolated, identified and characterized both microscopically and by biochemical assays. Each isolate was tested across a panel of antibiotics to determine their susceptibility profile. ESBL producing was confirmed using the double disk synergy test.
Isolation, identification and characterization
Inoculum from the 24 hours old broth culture were spread on the surface if a Salmonella-Shigella agar, incubated for 24 hours at 37°C ± 1°C. Pure colonies of the organisms were picked and grown by streaking method on a sterile petri dish containing 20ml of nutrient agar. The pure isolates were identified via the morphological appearance of their colonies, Gram stain reaction and by biochemical tests which includes indole, oxidase, catalase test, according to Cheesbrough [11].
Antibiotic susceptibility testing
Susceptibility test for each isolate was performed following the disc diffusion method, as recommended by the National Committee for Clinical Laboratory Standards (CLSI, 2006), using Muller Hinton agar (MHA). The isolate was sub-cultured, standardized to 0.5 MacFarland and then tested using MHA agar against a multi- antibiotic disk containing amoxicillin + clavulanic acid (30μg), ceftazidime (30μg), ofloxacin (5μg), cefixime (5μg), ciprofloxacin (5μg), gentamicin (10μg), nitrofurantoin (200μg), and cefuroxime (30μg).The susceptibility of the isolates to each antibiotic was shown by a clear zone of growth inhibition. The inhibition zone diameter (IZD) was measured using a rule and interpreted using a CLSI, 2016 breakpoints.
Preliminary screening for Extended Spectrum β-LACTAMSE (ESBL) production
Third-generation Cephalosporins; cefotaxime (30g), ceftazidime (30µg), ceftriaxone (30µg), Aztreonam (30µg) and cefotetan (30µg) were placed on inoculated Mueller-Hinton Agar (MHA). Strains of organisms that showed resistant to at least one of the third generation Cephalosporin used were subjected to Double Disc Synergy Test to confirm them as ESBL producers [12].
Double Disc Synergy Test (DDST)
Briefly, amoxicillin-clavulanic acid disk (30 μg) was aseptically placed at the center of a Mueller-Hinton (MH) agar already inoculated with the test bacterial isolates. Ceftazidime (30 μg) and cefotaxime (30 μg) single antibiotic disks were each placed adjacent to the central disk of amoxicillin-clavulanic acid at a distance of 15 mm. The plates were incubated at 37oC for 18hrs [13,14]. The test organism was considered to produce ESBL if the zone size around the antibiotic disc increased from 5mm above in the presence of a beta-lactamase inhibitor disc (amoxicillin 20μg and clavulanic acid 10μg).
Results
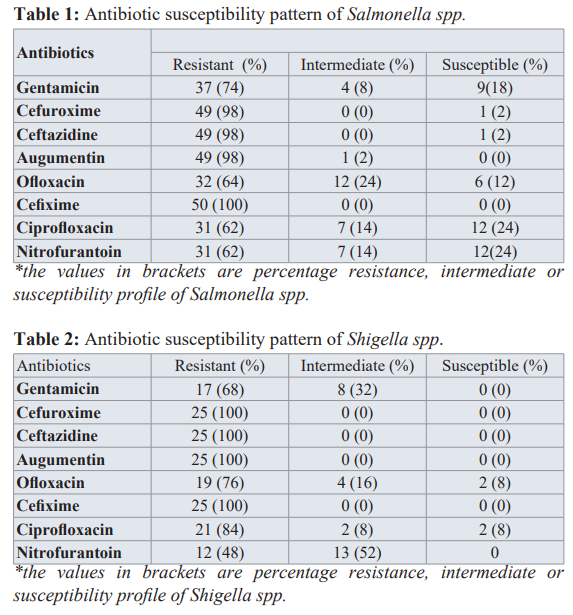
Total of 100 samples were collected within open market accounting for 60%, departmental stores 15% and 25% for other sources. Salmonella and Shigella isolates extracted, identified and characterized were 51 and 25 respectively. Of the 100 frozen chicken samples of collected, 51 isolates of Salmonella spp and 25 isolates of Shigella spp were isolated and cultivated using the Salmonella- Shigella agar. Morphologicallly, the Salmonella colonies appeared colorless with a black center while shigella colonies were just colorless. Microscopically, Salmonella and shigella appeared as slender red rods and as red rods respectively. The organisms were also identified by biochemical tests; Salmonella spp was catalase positive while Shigella spp was catalase-negative. Table 1 shows the antibiotic susceptibility profile of Salmonella spp .One hundred percent (100%) of Salmonella isolates were resistant to the cefixime (a third-generation cephalosporin), 98% were resistant to cefuroxime (a 2nd-generation cephalosporin), ceftazidime (3rd-generation cephalosporin) and amoxicillin-clavulanic and 64% resistant to ofloxacin. For shigella spp (Table 2) 100% of the isolates were resistant to all generation cephalospoin used; cefuroxime, ceftazidime, cefixime as well resistant to amoxicillin- clavulanic acid. Sixty-eight percentage, 68% of the isolates were resistant to gentamicin with 32% of isolates being intermediate. None of the isolates were susceptible to nitrofurantoin but then 52% of the isolate were intermediate while 48% were resistant. A 76% of the isolates were resistant to ofloxacin, 2% were susceptible while 4% were intermediate, 84% of the isolates were resistant, 2% susceptible and 2% intermediate to ciprofloxacin. Following preliminary test for ESBL 16 (31.3%) and 10 (40.0%) of Salmonella and Shigella isolates respectively were screen positives. However, none of the screen positive isolates were positive for ESBL.
Discussion
Contamination of frozen chicken meat by Extended Spectrum Beta-Lactamase (ESBL) producing bacteria may contribute to an increasing incidence of ESBL infections in humans. Food is very essential in health promotion and disease prevention. Poorly prepared and packaged street vended foods had been identified in many countries as causes of foodborne disease [15].
There are limited reports on the prevalence of shigellosis and enteric typhoid fever caused by consumption of a contaminated frozen chicken. Gottapu & Suresh, showed the presence of different serovars of salmonella isolated from cloacal swabs of live chicken and chicken dumps [16]. Obi& Ike, also reported Shigella strains isolated from cloacal swabs of chicken reared in Enugu [6].
Nitrofurantoin and ciprofloxacin proved to have the best activity. The result gotten from this study showed similar resistant patterns for cefuroxime, ceftazidime, and augmentin in a study conducted with Salmonella spp isolated from children at ile-Ife, Osun state [17]. Comparing the result of this study with a previous study carried out in Awka metropolis [18], there is a frightening increase in the resistant sttrains of Salmonella spp against third-generation cephalosporins.
The result obtained showed similar resistant patterns to amoxicillin-clavulanic acid but a wide but relatable difference in the resistant and susceptible patterns of other drugs in a previous study conducted in Nsukka, Enugu state [6]. However , Gu et al.[19] showed an increase in the pattern of resistance of shigella to third-generation cephalosporins.
After preliminary screening for ESBL using four third-generation cephalosporins and azetronam 16 (31.4%) and 11 (44%) of Salmonella spp and Shigella spp respectively were screen positive. However, all of the screen positive were confirmed to be ESBL negative using the double disk synergy test.
Conclusion
Of the 100 frozen chicken samples of collected, 51 isolates of Salmonella spp and 25 isolates of Shigella spp were isolated. Nitrofurantoin and ciprofloxacin proved to have the best activity against Salmonella spp among the antibiotics tested while the isolates shigella spp were resistant to all generation cephalospoins. The result of this study places a huge burden on the health of all consumers and lovers of frozen chicken in Awka. Thus care should be taken when handling frozen chicken and generally all meat products as this will go a long in reducing incidence of bacteria infection as well as development of resistance.
References
- Fagbamila IO, Barco L, Mancin M, et al. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS ONE. 2017; 12: 0173097.
- World Health Organization. Guidelines for the control of shigellosis, including epidemics due Shigella dysenteriae. 2005; 1.
- Kirk MD, Pires SM, Black RE, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015; 12: 1001921.
- McCrickard LS, Crim SM, Kim S, et al. Disparities in severe shigellosis among adults—Foodborne diseases active surveillance network, 2002–2014. BMC Public Health. 2018; 18: 221.
- Mehdi Dallal MS, Ranjbar R, Yaghoubi S, et al. Molecular epidemiology and genetic characterization of Shigella in pediatric patients in Iran. Infez Med. 2018; 26: 321-328.
- Obi O, Ike A. Occurrence and antibi gram Shigella spp in free range and intensively reared chicken in Nsukka, Enugu State, Nigeria. Microbiology Research Journal International. 2018; 25: 1-7.
- Phoebe CM Williams, James A Berkley. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Pediatrics and International Child Health. 2018; 38: 50-65.
- Li Y, Yang Q, Cao C, et al. Prevalence and characteristics of Salmonella isolates recovered from retail raw chickens in Shaanxi Province, China. Poultry Science. 2020; 99: 6031-6044.
- Makinde OM, Ayeni KI, Sulyok M, et al. Microbiological safety of ready-to-eat foods in low- and middle-income countries: A comprehensive 10-year (2009 to 2018) review. Comprehensive Reviews in Food Science and Food Safety. 2020; 30.
- Hur J, Jawale C, Lee JH. Antimicrobial resistance of Salmonella isolated from food animals: A review. Food Research International. 2012; 45: 819-830.
- Chessebrough M. District laboratory practice in Tropical countries. Part 1, Cambridge second Editions. Press Syndicate of the University of Cambridge. 2009.
- Chaudhary U, Aggarwal R. Extended spectrum B-lactamases (ESBL) - An emerging threat to clinical therapeutics. Indian Journal Med Microbiol. 2004; 22: 75-80.
- Ugwu MC, Edeani GI, Ejikeugwu CP, et al. Antibiotic Susceptibility Profile of Escherichia coli and Salmonella Causing Childhood Diarrhoea in Awka Municipality, South- eastern Nigeria. Clin Microbiol. 2017; 6: 277.
- Ejikeugwu C, Iroha I, Orji J, et al. Antibiogram of ESBL- Producing Pseudomonas aeruginosa Isolates of Nosocomial Origin. European Journal of Pharmaceutical and Medical Research. 2015; 2: 92-99.
- Mepba HD, Achinewhu SC, Aso SN, et al. Microbiological quality of selected street foods in Port Harcourt, Nigeria. Journal of Food Safety. 2007; 27: 208-218.
- Suresh Y, Bindu Kiranmayi CH, Srinivasa Rao T, et al. Multidrug resistance and ESBL profile of Salmonella serovars isolated from poultry birds and foods of animal origin. 2019; 8: 4.
- Japheh M, Olanran O, Asiwan H, et al. Isolaion ans evaluaion of Salmonella and Shigella spps in children in Ile-Ife, Nigeria. Int Clin Pathl J. 2016; 4: 1.
- Abuchi U, Gugu T, Ugwu BC, et al. Incidence and Antibiotic Susceptibility Pattern of Gram-Negative Bacteria Isolated from Aprons of Meat Vendors in Awka. Anambra Nigeria. 2016; 5: 269-277.
- Gu B, Zhou M, Ke X, et al. Comparison of resistance to third-generation cephalosporin’s in Shigella between Europe- America and Asia-Africa from 1998 to 2012. Epidemiology and Infection. 2015; 143: 2687-2699.