Appetite Control and Biotherapy in the Management of Autoimmune Induced Global Chronic Diseases
Author'(s): Ian James Martins1,2,3*
1Centre of Excellence in Alzheimer’s Disease Research and Care, Sarich Neuroscience Research Institute, Edith Cowan University, Verdun Street, Nedlands, Western Australia, Australia.
2School of Psychiatry and Clinical Neurosciences, The University of Western Australia, Nedlands, Australia.
3McCusker Alzheimer's Research Foundation, Hollywood Medical Centre, 85 Monash Avenue, Suite 22, Nedlands, Australia.
*Correspondence:
Ian Martins, School of Medical Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup, Western Australia 6027, Australia, Tel: +61863042574, E-mail: i.martins@ecu.edu.au.
Received: 17 April 2018; Accepted: 08 May 2018
Citation: Ian James Martins. Simona, et al. Appetite Control and Biotherapy in the Management of Autoimmune Induced Global Chronic Diseases. Clin Immunol Res. 2018; 2(1): 1-4.
Keywords
Editorial
Appetite control with relevance to immunometabolism has become critical to the treatment of non-alcoholic fatty liver disease (NAFLD), cardiovascular disease and diabetes [1-4]. The major defect in global chronic disease is autoimmune disease with defective adipose tissue and liver interaction involved with the release of inflammatory cytokines and adipocytokines [5] relevant to toxic immune reactions that involve the pancreas brain, heart, thyroid, kidneys and reproductive organs. Appetite control and autoimmune disease are connected with the identification of Sirtuin 1 (Sirt 1) as the anti-aging gene [1-3] involved in appetite regulation and the prevention of autoimmune disease [4].
Sirt 1 repression is responsible for mitophagy [4] in various cells and connected to autoimmune disease and irreversible programmed cell death in various cells and tissues. Science and medicine and its relevance to genomic medicine now need to consider Sirt 1 gene expression and its plasma Sirt 1 levels with relevance to accelerated immune reactions that trigger acute cardiovascular disease [6]. Sirt 1 is a nicotinamide adenine dinucleotide (NAD +) dependent class III histone deacetylase (HDAC) that targets transcription factors to adapt gene expression to metabolic activity and the deacetylation of nuclear receptors indicate its critical involvement in insulin resistance. In situ hybridization analysis has localized the human Sirt 1 gene to chromosome 10q21.3 [7].
The Sirt 1 gene has now been linked to autoimmune disease and various diseases with deletions, mutations, inversions and aberations in chromosome 10q21.3 [8-10]. Sirt 1 has a molecular weight (Mol Wt) of 81 kda with Mol Wt variation (81-110 kda) between laboratories. Sirt 1 is now identified as the heat shock gene [11,12] and involved in the deacetylation of heat shock factor 1 (HSF 1) and regulation of heat shock proteins (HSP) and nitric oxide metabolism connected to natural killer cell activity, mitophagy and autoimmune disease connected to chronic disease [13].
Sirt 1 is an acute phase protein involved with neuron proliferation [14] and its regulation of the suprachiasmatic nucleus is involved with control of the circadian rhythm [3]. The circadian rhythm and immune system are closely connected to the immune response [15-17]. Sirt 1 may now be considered to be involved with the circadian rhythm of the immune system and critical to the immune response in various communities. Sirt 1 is important to regulation of HSF1 and T and B cell immune response [18-20] and is involved with B cell antibody regulation and T cell tolerance [21,22]. Sirt 1 is involved in regulation of nuclear receptors peroxisome proliferator-activated receptor (PPAR) and liver X receptor (LXR) with relevance to metabolic homeostasis [3] and may now be connected to the regulation of these nuclear receptors (PPAR/ LXR) in inflammation and immunity [23]. Sirt 1 is now important to the immune mediated control of synaptic plasticity [24,25] and anti-epileptic drugs relevant to the immune system and epileptic stroke [26,27].
Laboratory evaluations using established and emerging techniques in immunology are essential for the diagnosis and management of immune-mediated disorders. Diagnosis of allergy, autoimmune disease serology, protein immunology including complement testing, cancer immunology, histocompatibility and immunogenetics, primary/secondary immunodeficiencies and infectious disease serology may now require analysis of a diagnositc protein that is critical for mitochondrial apoptosis with early interpretation of global health and chronic disease.
The progression of global chronic disease may now involve the measurement of plasma Sirt 1 for primary autoimmune disease with the critical inter-relationship between immunology tests and other diagnositic tests that involve biochemistry, microbiology, and hematology. Immunization, vaccinations and vaccine programs for various diseases may require specific measurements of plasma Sirt 1 levels. Sirt 1 analysis on blood, plasma and sera with other acute phase proteins (Figure 1) may be important to other immunological tests and collection of plasma/sera sensitive to Sirt 1 stability and associated with appropriate preservative, freeze/thaw conditions and long term storage conditions.
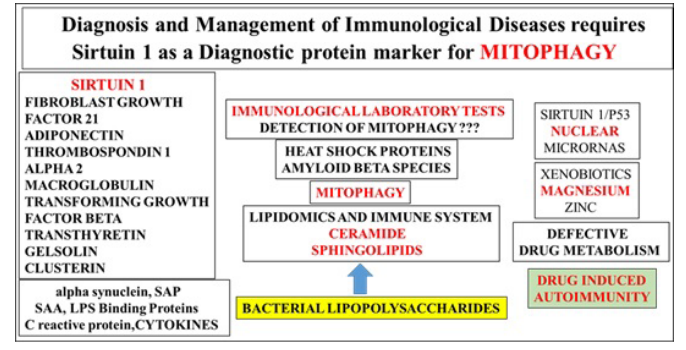
Figure 1: Measurements of specific plasma proteins and gene expression assays are required for assessement of mitophagy with connections to various immunological diseases.Analysis of Sirt 1 in the plasma and nucleus will allow early detection of automimmune disease as primary induction factor in global chronic disease. Lipidomic assays for immunogenic lipids may be required to determine programmed cell death. Heat shock protein plasma analysis may provide information with relevance to natural killer cell activation in chronic disease. NAFLD and autoimmune disease are now connected with impaired hepatic drug metabolism associated with drug induced autoimmunity. Bacterial lipopolysaccharides, magnesium and zinc levels should be assessed and relevant to magnesium deficiency induced autoimmune disease.
Bacterial lipopolysaccharides and HSP 60, 70 and 90 need to assayed on samples to avoid misinterpretations with relevance to Sirt 1 repression and mitophagy [4,5,8] in immunological associated chronic diseases. Gene expression assays of nuclear Sirt 1 for assessment of autoimmune disease may be required [5] and associated with measurements of plasma Sirt 1 levels in various immunological diseases. Sirt 1 is connected to hepatic drug metabolism [28] and Sirt 1 repression may require Sirt 1 plasma analysis to determine drug induced immune hypersensitivity reactions (Figure 1).
Appetite control and nutritional regulation of Sirt 1 has become important to immunotherapy and the clinical treatment of global chronic diseases. Nutritional diets that contain Sirt 1 activators [25,27] have become vital to immunotherapy research to maintain immunometabolism and prevent mitophagy (Figure 1). Lipidomic analysis have identified immunogenic lipids such as ceramide and sphingosine 1 phosphate that induce apoptosis and programmed cell death [29-31]. Appetite control and nutritional intake is essential to prevent the generation of immunogenic lipids, maintain the heat shock gene Sirt 1 [32] with relevance to the generation of immunogenic heat shock proteins (HSP), activation of natural killer cells [5] and nitric oxide induced immunological alterations in humans [13].
Sirt 1 as an immunosenescene gene [13,32] is connected to the senescence of various immune cells and inflammatory responses in aging [33-35]. Appetite and diets that regulate plasma Sirt 1 [36] require plasma analysis of other Sirt 1 regulated protein hormones that are involved in the immune response and glucose homeostasis (Figure 2), such as adiponectin, fibroblast growth factor 21, brain derived neurotrophic factor, neuropeptide Y and apelin [3,37]. Insulin therapy is involved in regulation of the immune system [38,39] but Indian spice therapy (doses) for activation of immune system [40,41] should be carefully assessed with relevance to glucose therapy. Excessive Indian spice intake may interfere with insulin therapy [42] by increased cellular glucose uptake that may induce hyperglycemic mitochondrial apoptosis [42].
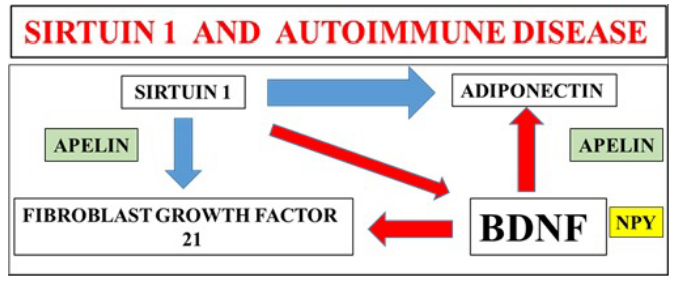
Figure 2: Sirt 1 regulates protein hormones such as adiponectin, fibroblast growth factor 21, neuropeptide Y and apelin involved in glucose homeostasis. These protein hormones are involved in the regulation of the immune system that involves autoimmune disease. Defective adipose tissue and liver interaction leads to defective regulation of these protein hormones with the induction of autoimmune disease and NAFLD relevant to various global chronic diseases. Appetite control that regulates Sirt 1 and these protein hormones reverse autoimmune disease with the prevention of NAFLD and reversal of various chronic diseases.
Conclusion
Human survival and the immune system are now relevant to the global chronic disease epidemic that involves NAFLD, cardiovascular disease and diabetes. Chronic disease assessment require measurement of plasma Sirt 1 levels with relevance to autoimmune disease and mitophagy. Biotherapy that involves immunotherapy may stabilize mitophagy that is connected to autoimmune disease and the early induction of global chronic diseases. Appetite control is essential to maintain Sirt 1 levels and to prevent the generation of immunogenic lipids and proteins that induce programmed cell death. Autoimmune disease and immunometabolism defects are now connected to the primary induction of mitophagy with the acceleration of chronic diseases in the developed and developing world.
Acknowledgment
This work was supported by grants from Edith Cowan University, the McCusker Alzheimer's Research Foundation and the National Health and Medical Research Council.
References
- Martins Appetite Control and Nutrigenomic Diets are connected to Immune Regulation and Diabetes Prevention. EC Nutrition. 2017; 12: 120-123.
- Martins IJ. Appetite Control with Relevance to Mitochondrial Biogenesis and Activation of Post- Prandial Lipid Metabolism in Obesity Linked Ann Obes Disord. 2016; 1: 1012.
- Martins IJ. Anti-Aging Genes Improve Appetite Regulation and Reverse Cell Senescence and Apoptosis in Global AAR. 2016; 5: 9-26.
- Martins Autoimmune disease and mitochondrial dysfunction in chronic diseases. Res Chron Dis. 2017; 1: 10- 12.
- Martins IJ. Defective Interplay between Adipose Tissue and Immune System Induces Non Alcoholic Fatty Liver Disease. Updates Nutr Disorders 2017; 1: 1-6.
- Martins Genomic Medicine and Acute Cardiovascular Disease Progression in Diabetes. Int J Medical Stud. 2018; 3: 124-130.
- Voelter-Mahlknecht S, Mahlknecht U. Cloning chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin Int J Mol Med. 2006; 17: 59-67.
- Martins IJ. The Future of Genomic Medicine Involves the Maintenance of Sirtuin 1 in Global Populations. Int J Mol 2017. 2: 1-4.
- Biason-Lauber A, Böni-Schnetzler M, Hubbard BP, et al. Identification of a SIRT1 Mutation in a Family with Type 1 Cell Metab. 2013; 17: 448-455.
- Hughes JW, Herold KC. Novel SIRT1 Mutation Linked to Autoimmune Diabetes in Cell Metab. 2013; 17: 311- 312.
- Martins IJ. Heat shock gene Sirtuin 1 regulates post-prandial lipid metabolism with relevance to nutrition and appetite regulation in diabetes. Int J Diab Clin Diag. 2016; 3: 20.
- Martins IJ. Type 3 diabetes with links to NAFLD and Other Chronic Diseases in the Western World. Int J Diab. 2016; 1: 1-5.
- Martins Antimicrobial activity inactivation and toxic immune reactions induce Epilepsy in human. J Med Discov. 2017; 2: 1-7.
- Herskovits AZ, Guarente L . SIRT1 in neurodevelopment and brain senescence. Neuron. 2014; 81: 471-483.
- Labrecque N, Cermakian N. Circadian Clocks in the Immune J Biol Rhythms. 2015; 30: 277-290.
- Habbal OA, Al-Jabri Circadian rhythm and the immune response a review. Int Rev Immunol. 2009; 28: 93-108.
- Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune Nat Rev Immunol. 2013; 13: 190-198.
- Gandhapudi SK, Murapa P, Zachary D, et HSF1 is activated as a consequence of lymphocyte activation and regulates a major proteostasis network in T cells critical for cell division during stress. J Immunol. 2013; 191: 4068-4079.
- Murapa P, Gandhapudi S, Skaggs HS, et Physiological fever temperature induces a protective stress response in T lymphocytes mediated by heat shock factor-1 HSF1. J Immunol. 2007; 179: 8305-8312.
- Rao R, Home T, Yacoub A, et Heat Shock Factor 1 Promotes NF-Kb and B-Cell Signaling in a Preclinical Model of Chronic Lymphocytic Leukemia. Blood. 2015; 126: 5297.
- Gao B, Kong Q, Kemp K, et Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2–mediated reversal of T-cell tolerance. Proc Natl Acad Sci U S A. 2012; 109: 899- 904.
- Wang Q, Yan C, Xin M, et al. Sirtuin 1 Sirt1 Overexpression in BaF3 Cells Contributes to Cell Proliferation Promotion Apoptosis Resistance and Pro-Inflammatory Cytokine Med Sci Monit. 2017; 23: 1477-1482.
- Glass CK, Ogawa Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006; 6: 44-55.
- Nisticò R, Salter E, Nicolas C, et Synaptoimmunology - roles in health and disease. Mol Brain. 2017; 10: 26.
- Martins Dietary Interventions Reverse Insulin and Synaptic Plasticity Defects Linking to Diabetes and Neurodegenerative Diseases. SL Nutrition and Metabolism. 2017; 1: 1-5.
- Beghi E, Shorvon S. Antiepileptic drugs and the immune Epilepsia. 2011; 3: 40-44.
- Martins IJ. Food Quality and Advances in Pharmacological Management Prevent Mitochondrial Apoptosis and Epilepsy Induced RRNS. 2018; 2: 7-9.
- Martins Sirtuin 1 a Diagnostic Protein Marker and its Relevance to Chronic Disease and Therapeutic Drug Interventions. ECPT. 2018; 6: 209-215.
- Ballou LR, Laulederkind SJ, Rosloniec EF, et al. Ceramide signaling and the immune response. Biochim Biophys Acta. 1996; 1301: 273-287.
- Cinque B, Di Marzio L, Centi C, et Sphingolipids and the immune system. Pharmacol Res. 2003; 47: 421-437.
- Baumruker T, Prieschl EE. Sphingolipids and the regulation of the immune Semin Immunol. 2002; 14: 57-63.
- Martins Biotherapy and the Immune System in Ageing Science. ASNH. 2018; 2: 29-31.
- Vicente R, Maussetâ?Bonnefont AL, Jorgensen C, et Cellular senescence impact on immune cell fate and function. Aging Cell. 2016; 15: 400-406.
- Lasry A, Ben-Neriah Senescence-associated inflammatory responses aging and cancer perspectives. Trends Immunol. 2015; 36: 217-228.
- Hoenicke L, Zender L. Immune surveillance of senescent cells- biological significance in cancer- and non-cancer Carcinogenesis. 2012; 33: 1123-1126.
- Martins The Limitations of Food Intake and Biomarkers in the Prevention of Chronic Diseases. NTNF. 2017; 1: 1-3.
- Martins Nutritional diets accelerate amyloid beta metabolism and prevent the induction of chronic diseases and Alzheimers disease. Photon ebooks. 2015; 1-48.
- Ergun-Longmire B, Marker J, Zeidler A, et al. Oral insulin therapy to prevent progression of immune-mediated type 1 Ann N Y Acad Sci. 2004; 1029: 260-277.
- Xiu F, Stanojcic M, Diao L, et Stress Hyperglycemia Insulin Treatment and Innate Immune Cells. Int J of Endocrinol. 2104; 1-9.
- Jagetia GC, Aggarwal Spicing up of the immune system by curcumin. J Clin Immunol. 2007; 27: 19-35.
- Tiwari R, Karthik K, Chakraborty S, et Immunomodulators in day to day life a review. Pak J Biol Sci. 2013; 16: 826-843.
- Martins Indian Spices and Biotherapeutics in Health and Chronic Disease. Health. 2018; 10: 374-380.