Assessing the Efficacy of Novel Oral Anticoagulants after Acute Coronary Syndrome
Author'(s): Hamza Mohamed QabiL, Ahmed Mohamed Ramzy, Mahmoud Shawky Abdelmoneum and Khedr Ahmed Abdelfattah*
*Correspondence:
Khedr Ahmed Abdelfattah, Department of Cardiology, Faculty of Medicine, Benha University, Egypt, E-mail: khedrahmed0@gmail.com
Received: 13 August 2018; Accepted: 04 September 2018
Citation: Hamza Mohamed QabiL, Ahmed Mohamed Ramzy, Mahmoud Shawky Abdelmoneum, et al. Effects of Light Therapy on Vascular Function in Patients with Diabetic Peripheral Neuropathy. Cardiol Vasc Res. 2018; 2(3): 1-6.
Abstract
Background: After an acute coronary syndrome (ACS), patients remain at risk of major adverse cardiovascular events (MACE), despite contemporary treatment including aspirin and clopidogrel. The risk of MACE may be secondary to thrombin, which remains elevated after ACS.
Objectives: This study aimed to evaluate the safety and indicators of efficacy of the novel oral direct factor Xa inhibitor (rivaroxaban).
Patients and Methods: In our study, we randomly assigned 100 patients with a recent ACS to receive the oncedaily dose of 2.5mg of rivaroxaban or placebo in addition to dual antiplatelet therapy for six months. The primary efficacy endpoint was a composite of death from cardiovascular events, myocardial infarction, and stroke. The
secondary endpoint was death from cardiovascular events, myocardial infarction, or stroke and the incidence of bleeding at six months.
Results: Rivaroxaban significantly reduced the primary efficacy endpoint, as compared with placebo, with respective rates of 4% and 22% (P=0.007). Regarding secondary endpoint, the 2.5mg dose of rivaroxaban reduced the rates of death from cardiovascular causes (0% versus 8%, P=0.041). As compared with placebo, rivaroxaban increased the rates of minor bleeding (12% vs. 2%, P=0.049), with no major or fatal bleeding had been recorded.
Conclusions: In patients with a recent acute coronary syndrome, rivaroxaban reduced the risk of the composite endpoint of death from cardiovascular causes, myocardial infarction, or stroke. Rivaroxaban increased the risk of minor bleeding but not the risk of major or fatal bleeding.
Keywords
List of Abbreviations
ACS: Acute coronary syndrome; MACE: Major Adverse Cardiovascular Events; NOAC: Novel oral anticoagulant; NSTEMI: Non St Segment Elevation Myocardial Infarction; PCI: Percutaneous Coronary Intervention.
Background
Acute coronary syndrome (ACS) continues to be associated with significant morbidity and mortality [1]. Major adverse cardiovascular events (MACEs) occur to the highest rates of the initial hospitalization and first 30 days after an ACS, this risk may be linked in part to the generation of thrombin which plays a major role in thrombus formation and platelet aggregation [2].
The MACE outcome can be defined as the composite of all-cause mortality, myocardial infarction, or stroke. Myocardial infarction can be defined as elevated cardiac biomarkers together with ischemic symptoms or ECG-changes (ST-elevation or depression, new left bundle branch block, or new Q-waves). Stroke can be defined as an acute onset of a focal neurological deficit of presumed vascular origin lasting for 24 hours or more and further categorized as hemorrhagic or ischemic after imaging [3].
Vitamin K antagonists (VKA) were the only class of oral anticoagulants available to clinicians, but they have important limitations that can outweigh these advantages, such as the slow onset of action, a narrow therapeutic window and an unpredictable anticoagulant effect [4]. VKA-associated dietary precautions, monitoring, and dosing adjustment to maintain the international normalized ratio (INR) within the therapeutic range, and bridging therapy, are inconvenient for patients, expensive, and may result in inappropriate use of VKA therapy [5].
Several new oral anticoagulants with more stable pharmacokinetic and pharmacodynamic profiles have been licensed for clinical practice [6]. Rivaroxaban, apixaban (direct factor Xa inhibitor), and dabigatran (direct thrombin inhibitor) are the most extensively evaluated novel anticoagulant agents currently [7]. Monitoring of coagulation profiles is not required, but patients should be followed up regularly to detect conditions that may lead to changes in the expected efficacy or safety [8]. Moreover, patients should be warned that reduced adherence or non-adherence to the treatment regimen could be fatal due to thromboembolic events.
Objective
This study aimed to evaluate the safety and indicators of efficacy of the novel oral direct factor Xa inhibitor (rivaroxaban).
Patients and Methods
This cross-sectional study was conducted in Mahalla cardiac center (MCC) and Benha hospital affiliated to Benha University from August 2017 to February 2018.
This study population included 100 patients presented with recent ACS and was subdivided into two groups:
Group A: fifty patients with ACS who received rivaroxaban 2.5 mg once daily in addition to dual antiplatelet therapy (aspirin and clopidogrel).
Group B: fifty patients with ACS who received placebo (control group) beside dual antiplatelet therapy. This study included patients that had an ST-segment elevation myocardial infarction (STEMI), a non ST-segment elevation myocardial infarction (NSTEMI), or unstable angina. Key exclusion criteria included any additional antiplatelet therapy other than aspirin &clopidogrel e.g. ticagrelor or prasugrel. planned PCI, treatment of vitamin K antagonist, recent stroke (within 12 months before randomization), Conditions associated with increased risk of bleeding e.g. anemia (a hemoglobin level of less than 10 gs per deciliter) ,thrombocytopenia (a platelet count of less than 90,000 per cubic millimeter), history of severe bleeding (clinically significant gastrointestinal bleeding within 12 months before randomization, and previous intracranial hemorrhage) and renal impairment (a creatinine clearance of less than 30 ml per minute at screening).
Baseline evaluation
- Full history taking and clinical examination.
- 12 lead ECG on admission and whenever indicated.
- Routine laboratory investigation including, random blood sugar, lipid profile, kidney function tests (urea and creatinine), liver enzymes (SGPT &SGOT), cardiac enzymes (troponin &CKMB), CBC, PT, PTT, INR.
- Transthoracic echocardiography.
Study endpoints
Primary endpoint: Six months adverse events including a composite of death from cardiovascular events, myocardial infarction and stroke.
Secondary endpoint: Death from cardiovascular events, myocardial infarction, stroke and the incidence of bleeding at six months.
Statistical analysis
The collected data were tabulated and analyzed using (Statistical Package for Social Studies) SPSS version 19. Categorical variables were presented as number and percentages while continuous variables were expressed as a mean ±standard deviation. Chisquare tests (X2) "Z" test and student "t" tests were used. The accepted level of significance of this work was stated P<0.05.
Results
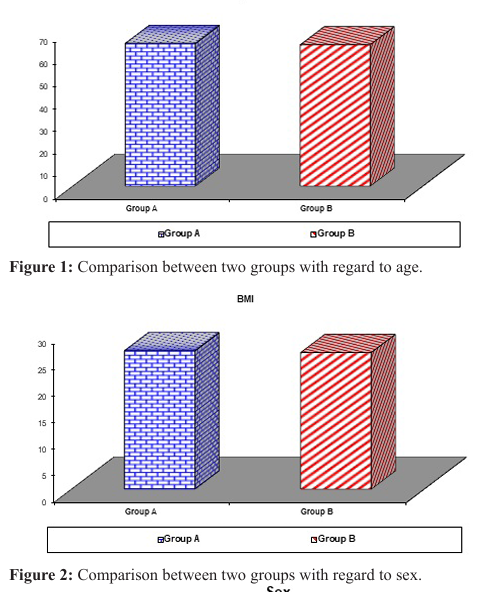
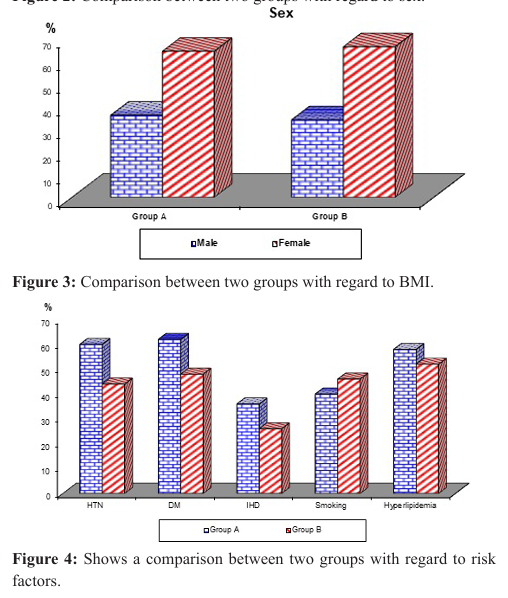
There was no significant difference between the two groups regarding age as the mean age of patients in group A was 63.56 ± 5.15 years and the mean age of patients in group B was 62.92 ± 5.99 years (p-value=0.568), also there was no significant difference between the two groups regarding sex distribution as 36% of patients in group A was males and 64% of the same group were females and group B included males constituting 34% of the group and females representing 66% of the group (p-value=0.834). Body mass index (BMI) showed no significant difference between the two groups as the mean BMI of patients in group A was 26.22 ± 1.82 and 25.86 ± 1.82 among patients in group B (p-value= 0.325) (Table 1), (Figures 1, 2, 3).
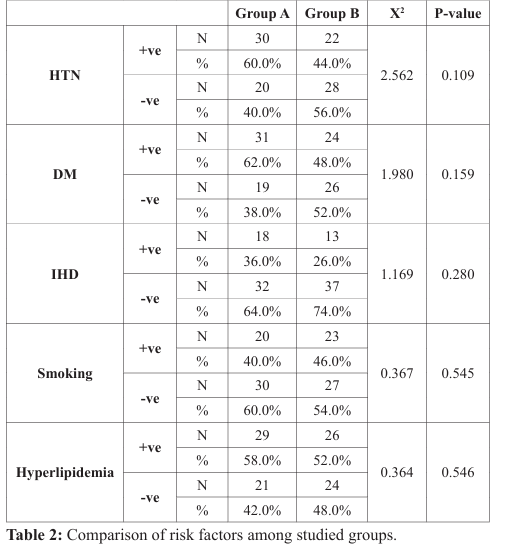
Comparison between the two groups regarding risk factors revealed no significant difference, as hypertension was in 60% of patients in group A and 44% of patients in group B (p-value=0.109), also diabetes mellitus was in 62% of patients in group A and 48% of patients in group B (p-value=0.159), smoking was in 40% of patients in group A and 46% of patients in group B (p-value=0.545) and dyslipidemia was in 58% of patients in group A versus 52% of patients in group B (p-value=0.546) (Table 2), (Figure 4).
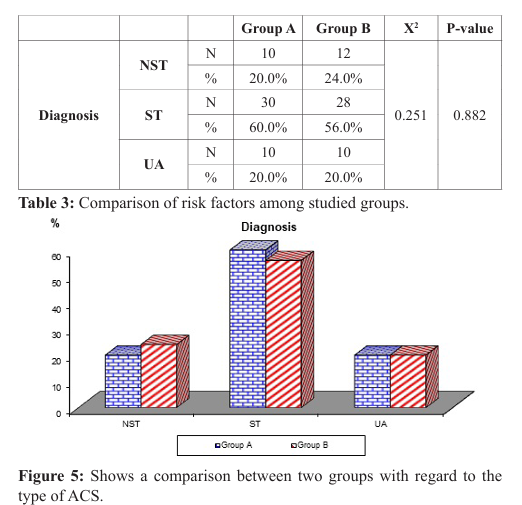
Regarding the type of acute coronary syndrome, the results showed no significant difference between the two groups as NSTEMI was present in 20% of patients in group A and 24 % of patients in the group B, while STEMI was present in 60% of patients in group A and 56% of patients in group B and unstable angina was present in 20% of patients in group A and 20% of patients in the group B (P-value=0.882 ) (Table 3), (Figure 5)
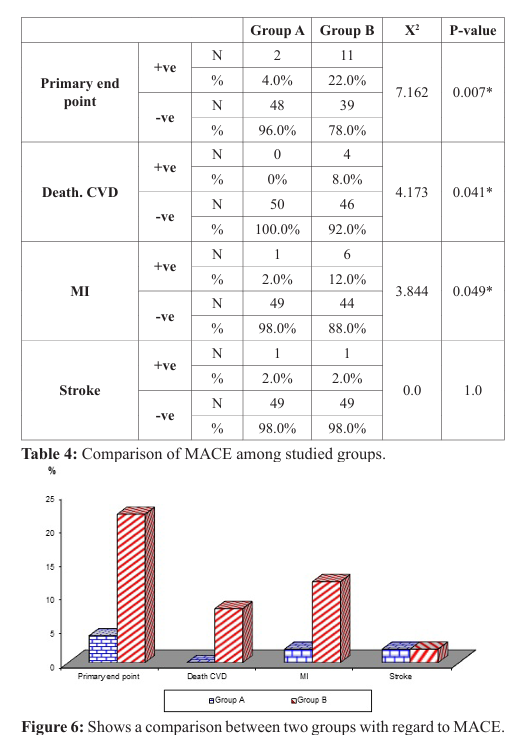
As regards primary efficacy endpoint of death from cardiovascular causes, myocardial infarction and stroke, six months follow up revealed a highly significant difference, as it was present in 4% of patients in group A while it was present in 22% in group B (P-value=0.007). Death from cardiovascular events represents 0% in group A and 8% in group B (p-value=0.041), MI represents 2% in group A and 12% in group B (p-value 0.042), stroke represent 2% in group A and 2% in group B (p-value=1) (Table 4), (Figure 6).
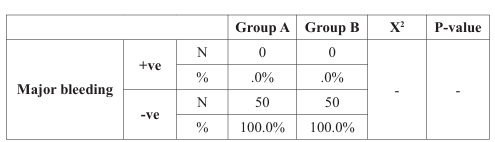
Regarding major bleeding and fatal bleeding, six months follow up data revealed that there was no major or fatal bleeding had been recorded in both groups. Minor bleeding was observed in 12% of patients in group A, while it was 2% in group B (p-value =0.049), and bleeding required medical attention occurred also in 12% (6 patients) in group A versus 2% (1 patient) in group B (p-value =0.049) (Table 5), (Figure 7).

Discussion
Coronary heart disease is responsible for more than half of all cardiovascular events in individuals less than 75 years of age. ACS refers to a group of conditions due to decreased blood flow in the coronary arteries such that part of the heart muscle is unable to function properly or dies [9]. The most common symptom is chest pain, often radiating to the left arm or angle of the jaw, pressurelike in character, and associated with nausea and sweating. The acute coronary syndrome usually occurs as a result of one of the problems: (STEMI, 30%), (NSTEMI, 25%) or unstable angina (38%) [10].
Platelets play a major pathogenic role in thrombus formation. Clopidogrel treatment in combination with aspirin can prevent recurrent ischemic events after ACS [11]. Despite treatment with dual-antiplatelet therapy, patients with stabilized ACS have an≈9% to 11% risk of suffering a recurrent adverse cardiovascular event within 1 year [12,13].
Rivaroxaban, another new factor Xa, and IIa inhibitors have been evaluated in patients after ACS. The phase 2 programs, which evaluated rivaroxaban, apixaban, dabigatran, and darexaban, all showed a dose-dependent increase in bleeding [14].
Studies have shown that thrombin levels stay elevated for months after an ACS event with a persistently elevated risk of adverse events despite antiplatelet therapy, so it is appealing to consider adding anticoagulation to the long-term care of patients with stabilized ACS to lower thrombin levels and improve outcomes [15].
In this study, we evaluated the safety and indicators of efficacy of the novel oral anticoagulant rivaroxaban (2.5mg once daily) after ACS in patients who remain at risk of recurrent ischemic events, despite contemporary treatment, including aspirin and clopidogrel.
As regard age, there was no significant difference between two groups, as the mean age of patients in group A was 63.56 ± 5.15 years and the mean age of patients in group B was 62.92 ± 5.99 years (p-value=0.568). Alhabiba and his colleagues in 2011 found that the average age of ACS presentation 58 ± 12.9 years in the study included 5055 patients [16].
As regard sex, there was no significant difference between two groups as 36% of patients in group A were males while 34% of group B were males (p-value=0.834). (Table1). Data from the Framingham Heart Study suggest that a harmful cardiovascular risk profile may be more cause than a consequence of age at menopause [17].
As regard risk factors, there was no significant difference between two groups, regarding diabetes mellitus, as 62% of patients in group A were diabetics while 48% of group B were diabetics (p-value=0.159). People with diabetes are at elevated risk for a number of serious health problems including cardiovascular disease and it is the most common complication associated with diabetes [18], Ahmed and his colleagues found in study aimed to assess the frequency of diabetes mellitus in patients with acute coronary syndrome 79 (31.6%) patients out of 250 patients of ACS who had diabetes [19]. Regarding smoking, 40% of patients in group A were smoker while 46% of group B was a smoker and regarding hypertension 30% of group A were hypertensive and 22% of group B were hypertensive (p-value=0.109). Aygul and his associates stated that hypertension and smoking are the major and potentially modifiable traditional risk factors that substantially increase the risk of developing Coronary heart disease [20].
In our study, regarding primary efficacy end point (death from cardiovascular causes, myocardial infarction, and stroke) in patients with a recent acute coronary syndrome six months follow up revealed that rivaroxaban significantly reduced the primary efficacy end point (2 patients in group A versus 11 patients in group B, p-value=0.007), and these findings are agree with both ATLAS-ACS 2–TIMI-51 trial in 2012 with two doses of 2.5 mg rivaroxaban, and ATLAS-ACS–TIMI-46 trial with two doses of 5mg rivaroxaban which showed that, treatment when added to antiplatelet therapy after a recent ACS [21,22], reduced primary efficacy end point, and disagree with APPRAISE-2 and RUBY-1 trials which showed non-significant reduction in the primary end point [23,24].
As regard death from cardiovascular events and myocardial (re) infarction, there was a highly statistically significant difference between the two groups, as death from cardiovascular events was in 0% patients of group A, while it was in 8% (4 patients) of group B (p-value=0.041), and MI was in 2% (1 patient) of group A, while it was 12% (6 patients) of group B (p-value=0.049), and this agrees with ATLAS ACS2-TIMI-51 and ATLAS ACS-TIMI-46, APPRAISE-1, and REDEEM trials which showed significant reduction in the rate of death from cardiovascular events and MI with using NOACs after ACS [21,22,26,27], and disagree with APPRAISE-2 and RUBY-1 trials which showed non-significant reduction in the rate of death from cardiovascular events and MI [23,24].
As regard stroke prevention after ACS, there was no statistically significant difference between the two groups, as the incidence of stroke was in 2% of patients in group A (1 patient), and it was the same in group B, and this agrees with ATLAS ACS 2–TIMI51 and APPRAISE-2 trials which revealed that rivaroxaban (2.5 mg bid) and apixaban (5mg bid) did not significantly reduce the incidence of stroke after ACS respectively [21,23].
Regarding major bleeding, six months follow up revealed that there was no major bleeding had been recorded in both groups, and these findings disagreed with ATLAS ACS 2–TIMI- 51, ATLAS ACS– TIMI-46, APPRAISE-1, RE-DEEM, APPRAISE-2 and RUBY-
1 trials which showed significant increase in the rate of major bleeding in patients who received NOAC, this difference between our study and the previous studies as regard major bleeding may be due to small sample size in our study and also lower dose of rivaroxaban (2.5mg once daily) [21-27]. Regarding fatal bleeding, also there was no fatal bleeding in both groups, and these findings agreed with ATLAS ACS 2-TIMI-51, ATLAS ACS-TIMI-46, APPRAISE-1 trials, which showed a non-significant difference between the two groups [21,22,25,26].
As regard minor bleeding and bleeding required medical attention, there was a statistically significant difference between the two groups, as minor bleeding was in 12% of patients in group A, while it was in 2% of group B (p-value =0.049), and bleeding required medical attention also was in 12% of patients in group A (6patient), while it was in 2% of group B (1 patients) (p-value =0.049), and this agrees with ATLAS ACS 2–TIMI 51 trial, ATLAS ACS–TIMI 46 trial, APPRAISE-2, RE-DEEM study and RUBY-1 trial which showed significant increase in the rate of minor bleeding and bleeding required medical attention with NOACs after acute coronary syndrome [21-24,27].
Study Limitations
- Small sample size.
- Short follow up.
- The incidence of adverse events (major and minor bleeding).
- Lack of randomization.
Conclusion
In patients with a recent acute coronary syndrome, rivaroxaban reduces the risk of the composite endpoint of death from cardiovascular causes, myocardial infarction, and stroke. Regarding safety, rivaroxaban increases the risk of minor bleeding but not the risk of major or fatal bleeding.
References
- Jneid H, Anderson JL, Wright RS, et al. ACCF/AHA focused update of the guideline for the management of patients with unstable angina non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012; 60: 645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. ACCF/AHA guideline for the treatment of STelevation myocardial infarction: the report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 127: e362-e425.
- Oldgren J, Wallentin L, Alexender J, et al, the New oral anticoagulant in addition to single or dual antiplatelet therapy after acute coronary syndrome. European Heart Journal. 2013; 34: 1670-1680.
- Ageno W, Gallus AS, Wittkowsky A, et al. American College of Chest Physicians Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012; 141: e44S-88S.
- Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J. 2012; 33: 1500-1510.
- Mismetti P, Laporte S. New oral antithrombotic: laboratory monitoring. J Thromb Haemost. 2010; 8: 621-626.
- Beyer-Westendorf J, Ageno W. Benefit-risk profile of non-vitamin K antagonist oral anticoagulants in the treatment of venous thromboembolism. Thromb Haemost. 2014; 16: 113-128.
- Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with nonvalvular atrial fibrillation. Europace. 2013; 15: 625-651.
- Amsterdam EA, Wenger N K, Brindis R G, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of American College of Cardiology/American Heart Association Task Force on Practice Guidelines".Circulation. 2014; 130: e344-e426.
- Torres M, Moayedi S. Evaluation of the acutely dyspnea elderly patient. Clin. Geriatr.Med. 2007; 23: 307-325, Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology, Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007; 28: 1598-1660.
- Barilla F, Pulcinelli FM, Mangieri E, et al. Clopidogrel plus indobufen in acute coronary syndrome patients with hypersensitivity to aspirin undergoing percutaneous coronary intervention. Platelets. 2013; 24: 183-188.
- Wiviott SD, Braunwald E, McCabe CH. TRITON-TIMI-38 study investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357: 2001-2015.
- Wallentin L, Becker RC, Budaj A, et al. PLATO trial investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361: 1045-1057.
- Algren, Budaj A, Granger CB, et al. Dabigatran versus placebo in patients with acute coronary syndrome on antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J 2011 May 7 (Epub ahead of print).
- Christersson C, Oldgren J, Block A, et al, the long-term treatment with ximelagatran, an oral direct thrombin inhibitor, persistently reduces the coagulation activity after a myocardial infarction. J Thromb Haemost. 2005; 3: 2245-2253.
- Alhabiba KF, Heredia A, Alfalfa H, et al. Baseline characteristics, management practices, and in-hospital outcomes in patients with acute coronary syndrome: Results of the Suadi project for assessment of coronary heart disease (SPACE).
- Maas AHEM, Appelman YEA. Gender differences in coronary heart disease. Netherlands Heart Journal. 2010; 18: 598-602.
- Goff DC, Gerstein HC, Ginsberg HN, et al. The Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007; 18: 4i-20i.
- Ahmed WH, Bittl JA, Braunwald E. Relation between clinical presentation and angiographic findings in unstable angina pectoris, and comparison with that instable angina. Am J Cardiol. 1993; 72: 544-550.
- Aygul N, Ozdemir K, Duzneli MA, et al. The comparative effects of long-term carvedilol versus bisoprolol therapy on QT dispersion in patients with chronic heart failure. Cardiology. 2009; 112: 168-173.
- Jessica L. Mega, Eugene Braunwald, Stephen D W, et al. Rivaroxaban in Patients with recent Acute Coronary Syndrome. The New England journal of medicine. 2012; 366: 9-19.
- Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACSTIMI 46): a randomized, double-blind, phase II trial, 4 July 2009; 374: 29-38.
- John H. Alexander, Renato D. Lopes, Stefan James, et al. Apixaban with Antiplatelet Therapy after Acute Coronary Syndrome.The New England journal of medicine. N Engl J Med. 2011; 365: 699-708.
- Gabriel Steg, Shamir R. Mehta, J. Wouter Jukema, et al. A randomized, double-blind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitors darexaban following acute coronary syndromes, European Heart Journal. 2011; 32: 2541-2554.
- Edward T. Carreras, Jessica L. Mega. Role of Oral Anticoagulants in Patients with an Acute Coronary Syndrome. Arterioscler Thromb Vasc Biol. 2015; 35: 520-524.
- Flores E, Wickemeyer W, Des Moines, et al. Antiplatelet therapy after acute coronary syndrome: results of the apixaban for apixaban, an oral, a direct, selective factor Xa inhibitor, in combination with prevention of an acute ischemic events (APPRAISE) Trial. APPRAISE Steering Committee and Investigators. 2009; 119: 2877-2885.
- Jonas Oldgren, Andrzej Budaj, Christopher B. Granger, et al. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial European Heart Journal. 2011; 32: 2781-2789.