Assessment of CD4+, CD8+ T cell and Th1 Cytokine Levels in Healthcare Workers Vaccinated against COVID-19 after the COVAX Initiative in Côte d’Ivoire
Author'(s): Adou Adjoumanvoulé Honoré1 , Yeboah Oppong Richard1, Kouacou Amah Patricia1 , Assi Aya Ursule1 , Memel Charline Roselle2, Seri Yida Jocelyne1, Moussa Salimata3, Oura Doris3, Koffi Adjo Mélissa4, Koya Hebert2, Bathily Maïmouna Sidibé5 and Dassé Sery Romuald1
1Immunology Laboratory, Medical Sciences, Felix HouphouëtBoigny University, Abidjan, Côte d’Ivoire.
2Immunology Laboratory, Medical Sciences, Alassane Ouattara University, Bouaké, Côte d'Ivoire.
3National Blood Transfusion Center, Abidjan, Côte d'Ivoire.
4Department of Immunology and Hematology, Cocody University Hospital Center, Abidjan, Côte d’Ivoire.
5Bingerville Mother-Child Hospital, Côte d’Ivoire
*Correspondence:
ADOU Adjoumanvoulé Honoré, Immunology Laboratory, Medical Sciences, Felix Houphouët-Boigny University, Abidjan, Côte d’Ivoire.
Received: 02 Jun 2023; Accepted: 09 Oct 2023; Published: 18 Oct 2023
Citation: Honoré AA, Richard YO, Patricia KA, et al. Assessment of CD4+, CD8+ T cell and Th3 Cytokine Levels in Healthcare Workers Vaccinated against COVID-19 after the COVAX Initiative in Côte d’Ivoire. Clin Immunol Res. 2023; 7(1): 1-8.
Abstract
The COVID-19 pandemic had urgently required the availability of effective vaccines to stop its spread. Despite reports of the effectiveness of these vaccines, SARS-CoV-2 continued to be transmitted. This raised concerns about the immune response to SARS-CoV-2. Two years after the COVAX initiative in our country, we conducted a study to assess the cellular response induced by vaccination among health workers in Abidjan. This was a cross- sectional study that included 350 health workers. It focused on age, sex, workstation, body mass index, history relating to COVID-19, existence of comorbidity, occupational stress, CD4+ T cell levels and CD8+ and the concentrations of the cytokines IFN-γ, TNF-α, IL-6 and IL-2. CD4+ and CD8+ T cell levels and cytokine titers were determined using the BD FACS CANTO II cytometer. Processing was performed using BD FACSCanto software and the CBA protocol. The population average was 40.65 years. CD8+ T-cell levels were significantly correlated with IFN-γ, TNF-α and IL-2 cytokine concentrations. A history of SARS-CoV-2 infection was significantly associated with CD8+ T cell and Th1 cytokine levels. In conclusion, in healthcare workers, T-cell levels continued to increase in the third trimester after vaccination against COVID-19. Additionally, history of SARS-CoV-2 infection appeared to stimulate the cytotoxic T cell response.
Keywords
Introduction
Discovered in December 2019, due to cases of pneumonia, SARS- CoV-2 led to an unprecedented COVID-19 pandemic. In response to this pandemic, a number of effective SARS-CoV-2 vaccines have been developed, evaluated and deployed in record time [1].
Studies results highlighted an efficacy of SARS-CoV-2 vaccines. In these studies, high levels of SARS-CoV-2 neutralizing antibodies and strong antigen-specific Th3 cellular responses were reported [2,3]. CD4 T cells cooperate with B cells to produce antibodies and orchestrate the response of other immune cells. CD8+ T lymphocytes kill infected cells to reduce the viral load. Several studies have reported T-cell activation in almost all subjects infected with SARS-CoV-2 [4-6]. SARS-CoV-2 specific CD4+ and CD8+ T cells are reported to have shown the best response against spike protein and produce Th3 effector cytokines (IFN-γ, TNF-α) in addition to Th3 cytokines (IL-4, IL- 5) and Th37 (IL-17). Th3-type cytokines tend to induce a pro-inflammatory response, while Th3-type cytokines induce an anti-inflammatory response [7]. However, the interaction mechanism of SARS- CoV-2 and the immune response induced are not sufficiently clear [8]. The accurate role of CD4 and CD8 T lymphocytes in the development or protection of COVID-19 is still poorly understood [9]. In addition, an increase in the number of cases of SARS- CoV-2 infection after full vaccination had been mentioned [10].
In Côte d'Ivoire, as part of the COVAX Initiative, four vaccine platforms were deployed throughout the country. These included inactivated whole virus vaccines (Sinovac-Coronavac), mRNA encapsulated in lipid nanoparticles (Pfizer-BNT162b2, Moderna- mRNA 1273) and adenoviral vectors (AstraZeneca-AZD1222, Janssen (Johnson & Johnson)-Ad26.COV2.S) [11]. Health workers who were at the forefront of COVID-19 management had paid a heavy price [12-14]. Like other countries, Côte d'Ivoire has opted for targeted vaccination of its healthcare professionals who constitute a group at risk of infection [14,15]. Two years after the COVAX Initiative, as vaccination continues in our country, a question arises about the immune protection of these healthcare workers. What about the post-vaccination cellular immune response of these workers? We seem to have little, if any, data in Côte d'Ivoire. This study aimed to assess T-cell and Th3 cytokine level in healthcare workers vaccinated against COVID-19 in Abidjan. We determined the quantitative characteristics of SARS- CoV-2-specific T cells and the Th3 cytokine profiles correlated with this response. We then identified the possible parameters likely to influence the levels of these lymphocytes. This could provide valuable insights about the extent of immunity mediated by SARS-CoV-2-specific T cells in health workers in Abidjan.
Materials and Methods
Study type and population
This was a prospective, cross-sectional over three months. It was part of a large-scale project investigating the carriage and immunogenicity of SARS-CoV-2 in healthcare workers. Participants were recruited and sampled at three university hospitals in Abidjan after obtaining their informed consent. Based on the workstation, we determined three levels of exposure risk.
(i) Personnel at low risk of exposure: no contact with patients (administrative personnel, etc.); (ii) Personnel at intermediate risk: contact with an unknown or suspected COVID-19 patient; (iii) High-risk personnel: contact with known COVID-19 patients. The population for this study was established from a random sample of 350 health workers vaccinated against COVID-19 as part of the above-mentioned project.
Data collection
Epidemiological, clinical, and vaccine-related data were collected using a questionnaire. Blood samples were associated with it (whole blood and serum). This study included the following parameters: age, sex, workstation, body mass index (BMI), COVID-19 history (SARS-CoV-2 infection, vaccination status, name of SARS- CoV-2 vaccination, time between SARS-CoV-2 infection and blood collection, time between vaccination and blood collection), presence of comorbidity (asthma, diabetes, hypertension, sickle cell disease, etc.), the existence of work-related stress due to COVID-19, CD4 and CD8 LT levels and concentrations of the cytokines IFN-γ, TNF-α, IL-6 and IL-2. The history of SARS- CoV-2 infection was justified by the result of a positive RT-PCR test (Reverse Transcription followed by a Polymerase Chain Reaction). Vaccination status and names of vaccines were obtained by checking the agent's vaccination record. Professional stress was assessed by using “The job content questionnaire of KARASEK with 26 items” [16].
Tests carried out
CD4+ and CD8+ T cell levels and cytokines titers were performed using BD FACS CANTO II cytometer (Becton, Dickinson and Company, BD Biosciences, San Jose, CA 95131 USA, Serial Number: V3389002039). The BD CD3/CD8 and CD3/CD4 assays and the 'BD™ CBA Human Th3 Cytokine Kit' comprising three groups of reagents were used.
(i) The Bead reagent (Human Capture Beads IL-2, IL-6, TNF, IFN-γ and Cytometer Setup Beads),
(ii) Antibody and standards reagent (Human Th3 PE Detection Reagent, Human Th3 Cytokine Standards, PE Positive Control Detector and FITC Positive Control Detector) and
(iii) Buffer reagents (Wash Buffer, Assay Diluent and Serum Enhancement Buffer). Lymphocyte count began with lysis of whole blood using BD FACS Lysing Solution, then samples were prepared for immunostaining. Sample processing and analysis was performed using BD FACSCanto software. To determine Th3 cytokine concentrations, we applied the CBA (Cytometric Bead Array) protocol. The principle is based on a method of capturing a soluble analyte or a set of analytes with beads of known size and fluorescence, enabling analytes to be detected using flow cytometry [17]. The CBA protocol was carried out in three steps: (i) preparation of the standards with Human Th3 Cytokine Standards,
(ii) preparation of the Mixing Beads with Human Th3 Cytokine Capture Beads reagents and (iii) dilution of the samples. Sample assays and cytometer acquisition were performed on the BD FACS CANTO II. Samples were processed using BD FACSDiva and FCAP Array software. Figure 1 shows the mean fluorescence intensity (MFI) of cytokines.
Ethics approval
This study was approved by « Comité National d’Ethique des Sciences de la Vie et de la Santé (Reference N°: 007-22/ MSHPCMU/CNESVS-km) »
Statistical analysis
Statistical analysis was carried out using SPSS V29.0 software.
Descriptive and analytical statistical methods were carried out according to the types of variables. Pearson correlation was used to compare two quantitative variables. In cases where the variance is equal and the observations are normally distributed, we used the Student T-test and the Anova test to compare the means of a quantitative variable and a categorical variable. In cases of inequality of variance, we used Mann-Whitney U test. XLSTAT 2023 was used for linear regression. Graphs were obtained using XLSTAT and GraphPad Prism version 9. A p-value Ë? 0.05 was considered as a statistically significant difference.
Results
Descriptive study
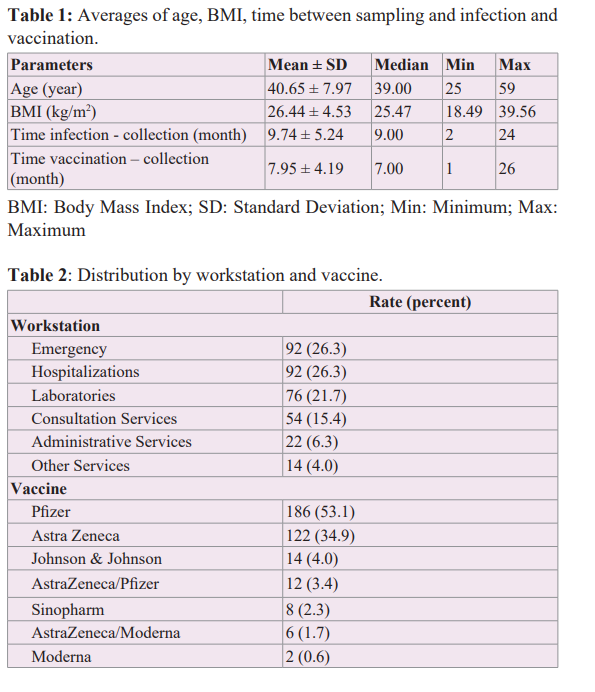
The average age was 40.65 years and 54.3% of the participants were overweight with an average BMI of 26.44 kg/m2. Participants sample was collected on average during the third trimester after vaccination (Table 1). Emergency departments and inpatient departments had the highest number of workers. Pfizer and AstraZeneca vaccines were the most administered vaccines in our population (Table 2).
Multiple Correlations
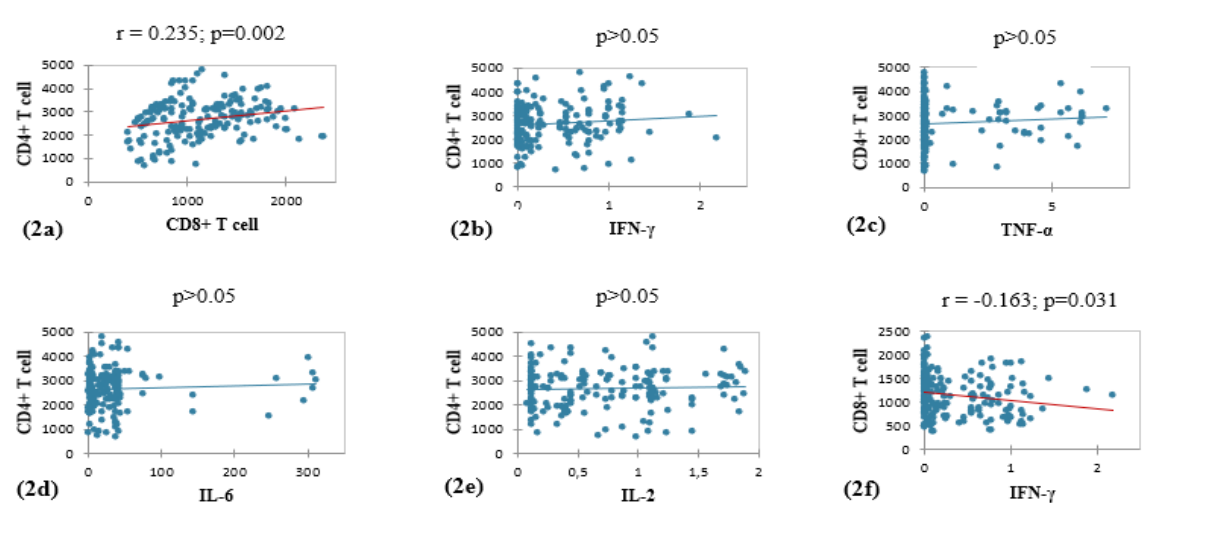
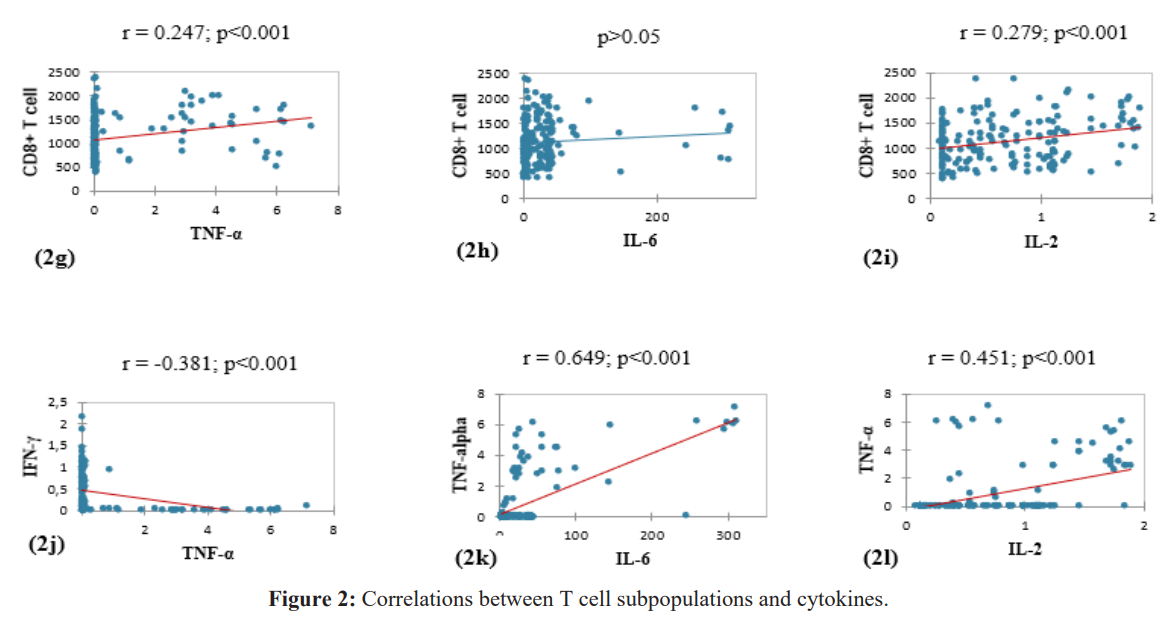
We observed a significant positive correlation between CD4+ and CD8+ T cell levels (Figure 2a). CD8+ T cell concentrations were significantly correlated with those of the cytokine IFN-γ, TNF-α, and IL-2 (Figures 2f, 2g, 2i). The level of TNF-α was significantly correlated with the concentrations of IFN-γ, IL-6 and IL-2 (Figures 2j, 2k, 2l).
Affecting Factors Study
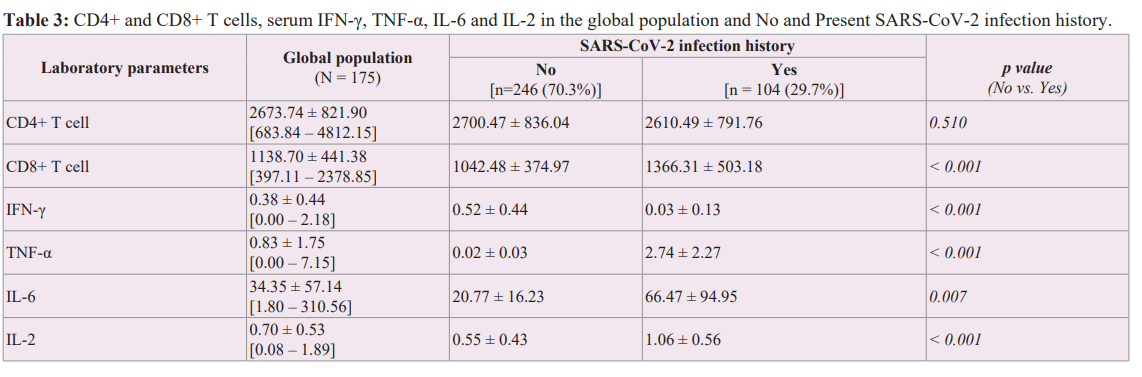
Comparison of means between T cells and cytokines with SARS-CoV-2 infection history.
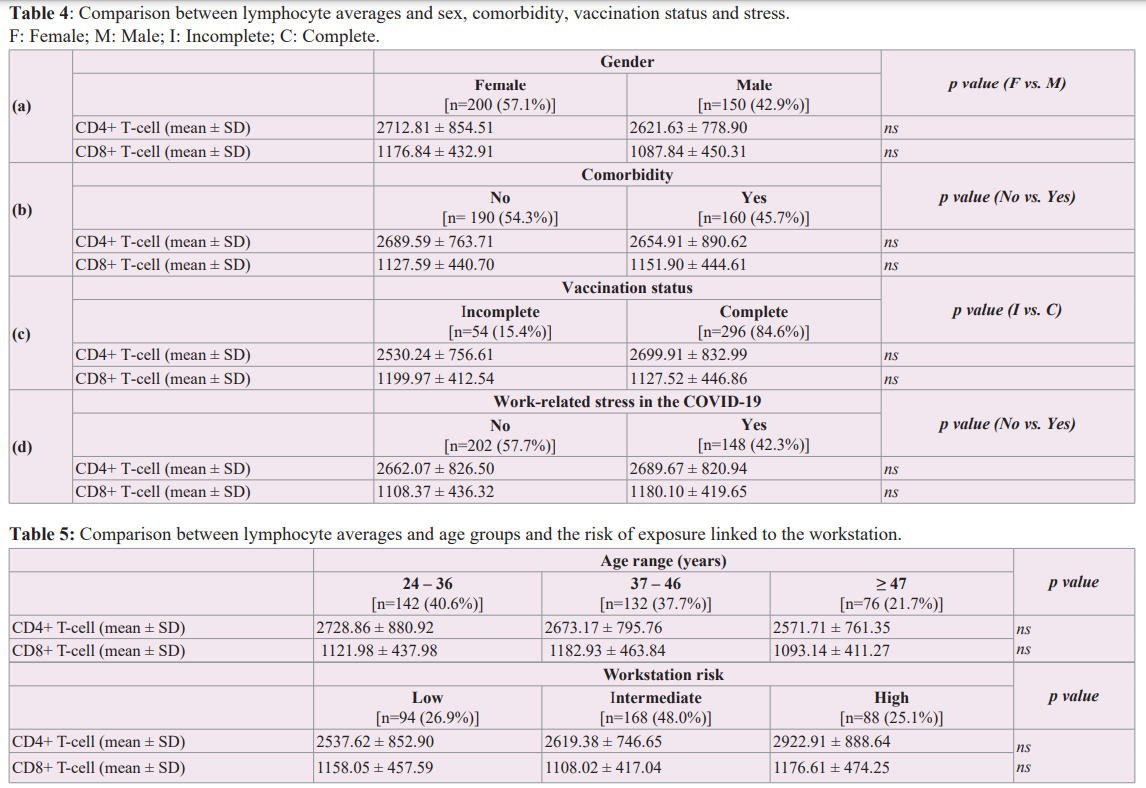
Besides CD4+ T cells, we observed a significant relationship between history of SARS-CoV-2 infection and CD8+ T cell and Th3 cytokines (Table 3). Linear regression of CD8+ T cell levels (dependent variable) by history of SARS-CoV-2 infection (explanatory variable) showed a significant correlation between the two variables. Only 11% of the variability in CD8+ T-cell count was explained by a history of SARS-CoV-2 infection. However, the information provided by the explanatory variable is significantly better than what would be explained by the average level of CD8+ T cells (Figure 3).
Comparison of means between T cells and BMI groups, and other parameters
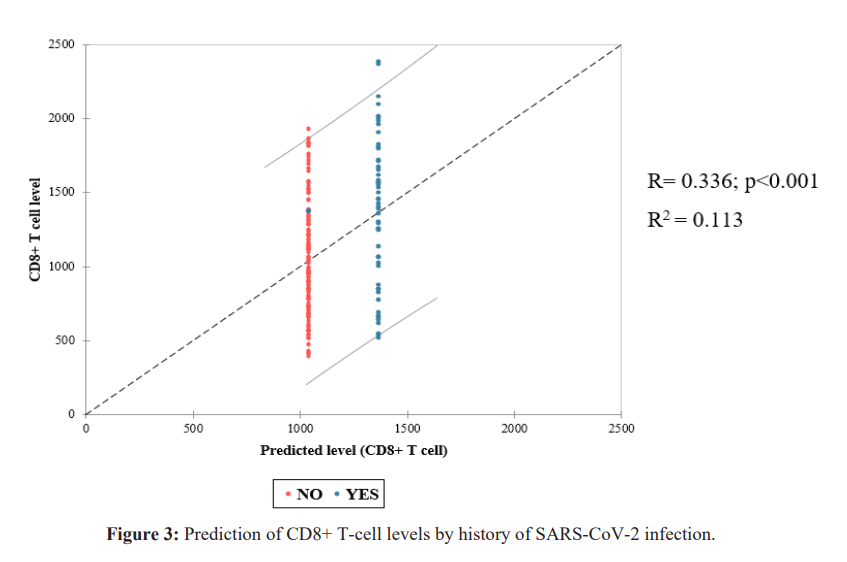
Comparing T-cell averages, we noted a significant link between CD4+ T-cell levels average and body mass index (Figure 4). However, there was no correlation between CD4+ lymphocyte count and BMI (Figure 5). For the other variables included in this study, in comparison with mean lymphocyte concentrations, we found no significant relationship. We noted a female predominance, an absence of stressors and comorbidity in most of our study population. Vaccination was complete in 84.6% of agents (Table 4). Our population was mostly young with a moderate risk of exposure to COVID-19 (Table 5).
Discussion
As the first available vaccines against SARS-CoV-2 were licensed, healthcare workers became a key target for immunization programs around the world. Two years after the COVAX Facility initiative in Côte d'Ivoire, we wanted to help identify factors that might influence the cellular response to the anti-SARS-CoV-2 vaccine in African subjects. Indeed, following the general model of the adaptive immune response, vaccination should help to control and/or prevent reinfection with SARS-CoV-2. Overall, our study supports the observation of other authors regarding the cellular response following vaccination against SARS-CoV-2 [2,3]. Studies have linked the immunogenicity of various vaccines to the degree of protection against infection or disease [18]. A report including health care workers who were candidates for vaccination in Nigeria, reported a high prevalence of history of SARS-CoV-2 infection (44%) [19]. We recorded a lower percentage (29.7%). Apart from the lack of association with mean CD4+ T-cell levels, we found significant associations between history of SARS- CoV-2 infection compared to mean CD8+ T-cell levels and most pro-inflammatory cytokines. In addition, we observed significant correlations between CD4+ and CD8+ T cell levels on the one hand, and between CD8 T cells and the main Th3 cytokines on the other. CD4+ T cell responses play an important role in the induction of cellular and humoral responses. Our results suggest that COVID-19 vaccines induce a coordinated cellular response by CD4+ T cells, which secrete Th3 cytokines to stimulate and activate CD8+ T cell cytotoxicity. Cytokines are protein mediators that provide critical signals for cell proliferation and inflammation [20]. Healthcare workers with a history of SARS-CoV-2 infection developed a greater cytotoxic CD8+ T response. Vaccination appears to enhance cytotoxic cellular immunity in these individuals. In our series, we did not observe significant difference between the different vaccines. Most vaccines target the SARS-CoV-2 spike protein S. There was no significant association between vaccine status and cellular response in our study. However, studies have shown that the first vaccine dose induces Spike-specific CD4+ T cell responses capable of producing IL-2, IFN-γ and TNF-α [21- 23]. In contrast, CD8+ T cell responses become more evident after the second dose [21]. Several factors may contribute to the heterogeneity of immune responses to SARS-CoV-2 [24]. Our population was moderately overweight on average. Agents with a normal body mass index appeared to develop a greater CD4+ T cell response. Reports on the humoral response mention a significant link between BMI and anti-SARS-CoV-2 antibody concentrations [25,26]. Apart from BMI, all parameters included in our series, especially age, comorbidity, and stress, showed no significant relationship with the level of T cell response. A previous study on adaptive immune responses induced by the anti-SARS-CoV-2 mRNA vaccine reported an association between age and T-cell response [22]. Other studies have also linked these parameters to the immune response, in particular the humoral response. In a report on healthcare workers after vaccination with BNT162b2 mRNA against COVID-19, Terpos et al. observed that female sex and young age are predisposed to a more intense immune response [27]. Pellini et al. reported a more intense humoral response in among young and females following vaccination with the BNT162b2 vaccine in healthcare workers [25]. People with diabetes, hypertension or hematologic disease have been reported to have reduced immune response after vaccination [28,29]. An association between stress and reduced immune defences has been reported, but the mechanisms involved remain unclear [30-32].
This study has some limitations that would have further elucidated the cellular vaccine response to SARS-CoV-2. The sample size may have influenced the results obtained. Although our sample was collected an average of 8 months after vaccination, a long- term longitudinal study would place more emphasis in assessing the persistence of the cellular response. Study of memory T lymphocyte subpopulations would have provided an insight into the level of protection afforded by vaccination in healthcare workers. The lack of analysis of Th3 profile cytokines also does not allow the assessment of the cooperation between T and B lymphocytes.
Conclusion
The results of this study showed that after vaccination with COVID-19, T-cell levels continued to increase during the third trimester. A history of SARS-CoV-2 infection appeared to enhance the cytotoxic T cell response. Ongoing observational studies are needed to determine: (i) Whether durable protection can be achieved; (ii) How long T cells can provide a durable protection; (iii) And whether there is a need to boost vaccination with COVID-19. It is also necessary to monitor the long-term immunity in healthcare workers.
Acknowledgments
Authors would like to thank « FONSTI » for the funding of the initial project, which underlies this study and, all the healthcare workers who voluntarily accepted to be part of this study.
References
- Addetia A, Crawford KHD, Dingens A, et al. Neutralizing Antibodies Correlate with Protection from SARS CoV2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J Clin Microbiol. 2020; 58: e02107-e02120.
- Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly specific T cells in humans. Nature. 2021; 595:572-577.
- Xu K, Dai L, Gao GF, et al. Humoral and cellular immunity and the safety of COVID 19 vaccines a summary of data published. Int Immunol. 2021; 33: 529-540.
- Arunachalam PS, Wimmers F, Mok CKP, et al. Systems biological assessment of immunity to mild versus severe COVID 19 infection in humans. Science. 2020; 369:1210-1220.
- Braun J, Loyal L, Frentsch M, et al. SARS CoV 2 reactive T cells in healthy donors and patients with COVID 19. Nature. 2020; 587: 270-274.
- Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell Responses to SARS CoV 2 Coronavirus in Humans with COVID 19 Disease and Unexposed Individuals. Cell. 2020;181: 1489-1501.
- dos Santos WG. Impact of virus genetic variability and host immunity for the success of COVID 19 vaccines. Biomed Pharmacother. 2021; 136: 111272.
- Cheng ZJ, Xue M, Zheng P, et al. Factors Affecting the Antibody Immunogenicity of Vaccines against SARS CoV 2 A Focused Review. Vaccines. 2021; 9: 869.
- Grigoryan L, Pulendran B. The immunology of SARS CoV 2 infections and vaccines. Semin Immunol. 2020; 50: 101422.
- Gallais F, Gantner P, Planas D, et al. Case Report Evolution of Humoral and Cellular Immunity in Two COVID 19 Breakthrough Infections After BNT162b2 Vaccine. Front Immunol. 2022; 13: 790212.
- MSHPCMU. Prévention Covid 19 Protégeons nous contre le coronavirus Ministre de la Santé et de l’Hygiène Publique Côte d’Ivoire. 2022.
- Mehta S, Machado F, Kwizera A, et al. COVID 19 a heavy toll on health care workers. Lancet Respir Med. 2021; 9: 226-228.
- Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID 19 among front line health care workers and the general community a prospective cohort study. Lancet Public Health. 2020; 5: e475-e483.
- https://www.euro.who.int/fr/health-topics/health-emergencies/coronavirus-covid-19.
- https://www.who.int/europe
- Questionnaire de Karasek Mesure du stress professionnel. 2022.
- Human Th3 Th3 Th37 CBA Kit. 2022.
- Naranbhai V, Garcia Beltran WF, Chang CC, et al. Comparative Immunogenicity and Effectiveness of mRNA 1273 BNT162b2 and Ad26. COV2 S COVID 19 Vaccines. J Infect Dis. 2021; 225: 1141-1150.
- Abdullahi A, Oladele D, Owusu M, et al. SARS COV 2 antibody responses to AZD1222 vaccination in West Africa. Nat Commun. 2022; 13: 6131.
- Florindo HF, Kleiner R, Vaskovich Koubi D, et al. Immune mediated approaches against COVID 19. Nat Nanotechnol. 2020; 15: 630-645.
- Arunachalam PS, Scott MKD, Hagan T, et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature. 2021; 596: 410-416.
- Bai J, Chiba A, Murayama G, et al. Sex Age and Ethnic Background Shape Adaptive Immune Responses Induced by the SARS CoV 2 mRNA Vaccine. Front Immunol. 2022; 13: 786586.
- Painter MM, Mathew D, Goel RR, et al. Rapid induction of antigen specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS CoV 2 mRNA vaccination. Immunity. 2021; 54: 2133-2142.
- Bertholom C. Réponse immunitaire associée au Sars CoV 2.Option Bio. 2021; 32: 15-17.
- Pellini R, Venuti A, Pimpinelli F, et al. Initial observations on age gender BMI and hypertension in antibody responses to SARS CoV 2 BNT162b2 vaccine. E Clinical Medicine. 2021; 36: 100928.
- Soffer S, Glicksberg BS, Zimlichman E, et al. The association between obesity and peak antibody titer response in COVID 19 infection. Obesity. 2021; 29: 1547-1553.
- Terpos E, Trougakos IP, Apostolakou F, et al. Age dependent and gender dependent antibody responses against SARS CoV 2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021; 96: E257-E259.
- Lustig Y, Sapir E, Regev Yochay G, et al. BNT162b2 COVID 19 vaccine and correlates of humoral immune responses and dynamics a prospective single centre longitudinal cohort study in health care workers. Lancet Respir Med. 2021; 9: 999-1009.
- Tawinprai K, Siripongboonsitti T, Porntharukchareon T, et al. Persistence of immunogenicity contributing factors of an immune response and reactogenicities after a single dose of the ChAdOx1 COVID 19 vaccine in the Thai population. Hum Vaccines Immunother. 2022; 18: 2035573.
- Jacque C, Thurin JM. Stress immunité et physiologie du système nerveux. Médecine sciences. 2002; 18: 1160-1166.
- Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003; 24: 444-448.
- Wieduwild E, Girard Madoux MJ, Quatrini L, et al. β2 adrenergic signals downregulate the innate immune response and reduce host resistance to viral infection. J Exp Med. 2020; 217.