Atrial Fibrillation, When and What are the Optimal Blood Pressure Values to Prevent it?
Author(s): Carlos Alberto Paterno Marchioli*
Fellow of the European Society of Cardiology, Laboratory of Cardiovascular Research, Castiglion Fiorentino (Tuscany), Italy
*Correspondence:
Carlos Alberto Paterno Marchioli, Fellow of the European Society of Cardiology, Laboratory of Cardiovascular Research, Castiglion Fiorentino (Tuscany), Italy, E-mail: carlos_paterno@ tiscali.it
Received: 25 Jun 2024; Accepted: 13 Aug 2024; Published: 21 Aug 2024
Citation: Paterno Marchioli CA. Atrial Fibrillation, When and What are the Optimal Blood Pressure Values to Prevent it?. Cardiol Vasc Res. 2024; 8(2): 1-7.
Abstract
Hypertensive Cardiovascular Disease is a complex, multifactorial, and chronic illness, frequently beginning during childhood or adolescence, could develop functional and/or structural cardiovascular organ damage by the increase of the volume/pressure level and the arterial stiffness, even before it exceeds the “normal” systolic and diastolic blood pressure levels. In the last decades, the pulsatile arterial function has been increasingly recognized as a major determinant of cardiovascular risk. It is known that hypertension is the most prevalent independent risk factor for atrial fibrillation in the population. Both structural remodelling and subsequent electrophysiological changes by atrial fibrosis constitute an arrhythmogenic substrate for the induction and maintenance of atrial fibrillation.
Atrial fibrillation has been associated with stroke, heart failure, and cardiovascular mortality. The research of non-antiarrhythmic drugs is a new and very interesting field of challenges to prevent atrial fibrillation onset and control recurrences.
The study aimed to know if there is some limit of age to make an effective treatment, and which systolic and diastolic values are necessary to achieve to stop atrial fibrillation.
Keywords
Background
Hypertensive Cardiovascular Disease is a complex, multifactorial, and chronic illness, frequently beginning during childhood or adolescence, could develop functional and/or structural cardiovascular organ damage by the increase of the volume/ pressure and the arterial stiffness, even before it exceeds the “normal” systolic and diastolic blood pressure levels [1,2].
In the last decades, the pulsatile arterial function has been increasingly recognized as a major determinant of cardiovascular risk. It is known that Hypertensive Cardiovascular Disease, frequently named arterial hypertension, is the most prevalent independent risk factor for atrial fibrillation in the population. Both structural remodelling and subsequent electrophysiological changes by atrial fibrosis constitute an arrhythmogenic substrate for the induction and maintenance of atrial fibrillation. Atrial fibrillation has been associated with stroke, heart failure, and cardiovascular mortality. It has been shown that even in the normotensive range, the incidence of cardiovascular disease progressively increases from optimal to normal to high-normal blood pressure values [3].
“Normal” blood pressure is the threshold that does not cause cardiovascular damage, even today we are far from knowing the true limit values. Therefore, to diagnose arterial hypertension, the actual cut-off values of systolic and diastolic blood pressure could be relatives.
The last Guidelines on arterial hypertension of the European Society of Hypertension and the International Society of Hypertension (2018) fixed the new limits into 130/80 mmHg, [4] but maybe it is not definitive probably because it could be less.
Atrial Fibrillation is a frequent complication of arterial hypertension, coronary heart disease, and valvular heart disease, it is the most frequently encountered arrhythmia in clinical practice with a global prevalence of 2-3%, furthermore it involves an increased risk of stroke and/or heart failure, and in particular of sudden cardiac death [5-7].
It was Middlekauff et al. first to report an increased risk of sudden cardiac death in patients with atrial fibrillation associated with advanced heart failure [8]. The pathophysiological mechanism has not yet been well defined [2]. Atrial fibrillation is a frequent complication of arterial hypertension affecting approximately 9 million European citizens aged >55 years in 2010 and with prevalence estimates exceeding 18 million by 2060 [9].
Harmful effects of angiotensin
Angiotensin II is undoubtedly one of the most powerful vasopressor substances known, it is able to regulate cardiac contractility [10], cell coupling and impulse propagation [11-14], as well as being responsible for remodeling and induction of apoptosis [15,16].
Furthermore, Angiotensin II stimulates collagen synthesis, extracellular matrix remodeling, vascular hypertrophy, increases oxidative stress and reduces elastin production in the vascular wall. The angiotensin receptor blockers (ARBs), have been shown to be very effective in controlling blood pressure and reducing cardiovascular morbidity and mortality and as such, ARBs have been recommended in all the arterial hypertension treatment guidelines.
An interaction between angiotensin II and aldosterone has been demonstrated, since some of the cellular effects of angiotensin II occur through aldosterone-dependent pathways, [17] for example, Angiotensin II and aldosterone synergistically induce smooth muscle cell proliferation [18].
Harmful Effects of Aldosterone
Aldosterone is now considered a major cardiovascular-risk steroid hormone, it exerts its effects through genomic and nongenomic pathways. The harmful effects of aldosterone are innumerable particularly in presence of salt:
Increase sympathetic nervous system activation [19], myocardial calcium channel expression, and myocardial apoptosis, produce glucose intolerance and insulin resistance, decrease sodium excretion and increase the filling pressure by water retention, impairs endothelial function by oxidative stress which would alter diastolic function by myocardium stiffness and induce to heart failure status, produce inflammation that contributes to the development of renal, left ventricular interstitial, and atrial fibrosis with subsequent remodeling-induced electrical instability that promotes the development of arrhythmias such as new-onset atrial fibrillation [20-25].
Arrhythmias developed by hypokalemia and expression of myocardial calcium channels [26,27], increase atrial fibrosis with subsequent structural remodeling developing a substrate for atrial arrhythmias [28] from electrical instability promoting new-onset atrial fibrillation [29,30].
The phenomenon of "aldosterone escape" occurs even in the presence of a combination therapy with Angiotensin II Converting Enzyme Inhibitors and ARBs [31].
Changes in atrial electrical properties occur early in Hypertensive Cardiovascular Disease, preceding the appearance of left ventricular and left atrial enlargement [32], and this electrical instability was shown to promote the development of atrial fibrillation [29,30].
The antihypertensive effect of hypotensive drugs is consistently associated with a reduction in cardiovascular incidents and mortality in patients with hypertension [33]. Aldosterone receptor antagonism appears to be a potential therapeutic option for atrial fibrillation [30]. The research of non-antiarrhythmic drugs that act both on the electrical and mechanical atrial remodelling, that constitutes the substrate of arrhythmia, is a new and very interesting field of challenge to prevent its onset and to control recurrences.
Objective
The study aimed to know if there is some limit of age to start an effective treatment, and which systolic and diastolic values are necessary to achieve to stop the onset of atrial fibrillation.
Design & Methods
This cross-sectional and observational sub-study comes from a precedent study with 1500 patients divided into three groups of therapy: ARB + MRA, ARB alone, and ARB + hydrochlorothiazide [34]. For this study, 500 patients were enrolled, 328 women (65.6%), and 172 men (34.4%), diagnosed as hypertensive patients with normal kidney function, treated with the association of ARB + MRA, without the addition of anti-arrhythmic drugs, during 6 or more months of therapy, both sexes, initially divided into 4 groups.
Group A: (Without AF → With AF)
Patients with no history of atrial fibrillation episodes or brief phases of atrial fibrillation in the 24-hour Holter monitoring who during the above-mentioned therapy have recorded the onset of atrial fibrillation even in brief phases.
N = 0
Group B: (Without AF → Without AF)
Patients with no history of atrial fibrillation episodes or brief phases of atrial fibrillation in the 24-hour Holter monitoring who continued without atrial fibrillation episodes or brief phases in the 24-hour Holter during therapy.
N = 407 (81.4%)
Group C: (With AF → Without AF)
Patients with a history of atrial fibrillation episodes or brief phases of atrial fibrillation in the 24-hour Holter monitoring who have not had episodes of atrial fibrillation or brief phases in the 24-hour Holter monitoring during therapy.
N = 76 (15.2%)
Group D: (With AF → With AF)
Patients with a history of AF episodes or brief phases of atrial fibrillation in the 24-hour Holter monitoring who during therapy had episodes of atrial fibrillation or brief phases in the 24-hour Holter monitoring.
N = 17 (3.4%)
In this work we will refer only to hypertensive patients with and without complications of atrial fibrillation; we have not recorded patients with overt ischemic heart disease or valvular disease more important than minimal mitral, aortic, or tricuspid regurgitation.
Non-Invasive Arterial Tonometry
Central Haemodynamic Parameters were measured in mmHg.
Augmentation Index adjusted to 75 beats/min was measured in percentage. All data were recorded by the SphygmoCor PVX device (AtCor Medical, Inc, Australia), software version 9, a validated pulse wave analysis system using high-fidelity technique with appropriate computer software and a generalized transfer function, measured according to established protocols with operator index ≥85%. In addition, differences between observed values and normal levels of Augmentation Index (Diff-AIx) were assessed according to normal ranges for sex and age.
Statistical Analysis
Continuous variables were summarized using descriptive statistics. Data are expressed as mean + standard deviation. Categorical variables were summarized using patient counts and percentages. Differences between the means of systolic and diastolic blood pressure and central haemodynamic parameters in the treatment groups, both sexes, were compared using Student's t-test for independent samples. Statistical tests were two-sided and the alpha level was set at 0.05. All analyses were performed using SAS version 9.4 software.
Results
As can be seen in Table 1, no patients were registered in group The largest group was the group B. A decrease in systolic and diastolic blood pressure was found between the first and last register representing the beginning of therapy and the last value in the patient's medical record, in groups B, C, and D, both sexes. In no case was it necessary to suspend therapy in patients, except
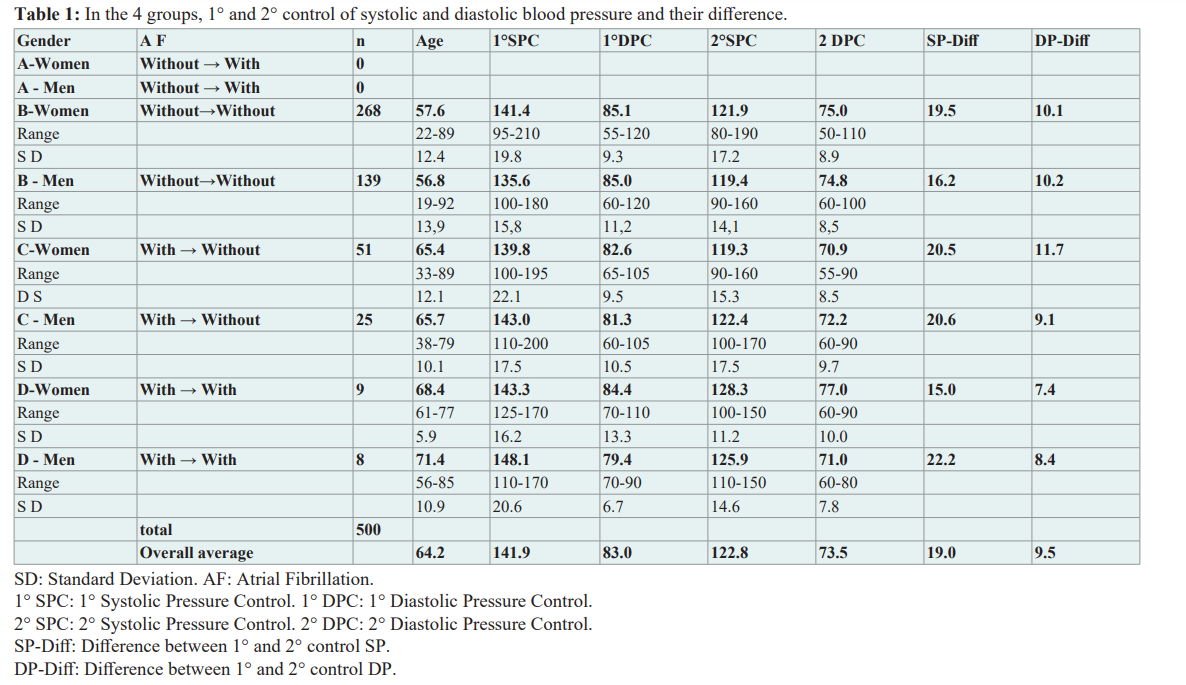
For some decreases from the initial dosage due to a slight increase in plasma potassium and/or creatinine which later regressed to normal values.
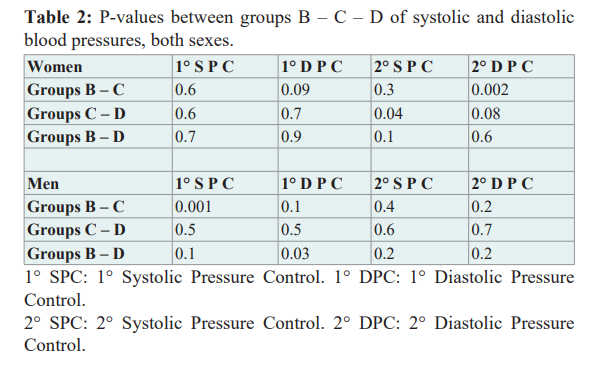
The first and the last measures of the systolic and diastolic values were compared into the three therapy groups, both genders, without a clear statistically significant difference Table 2.
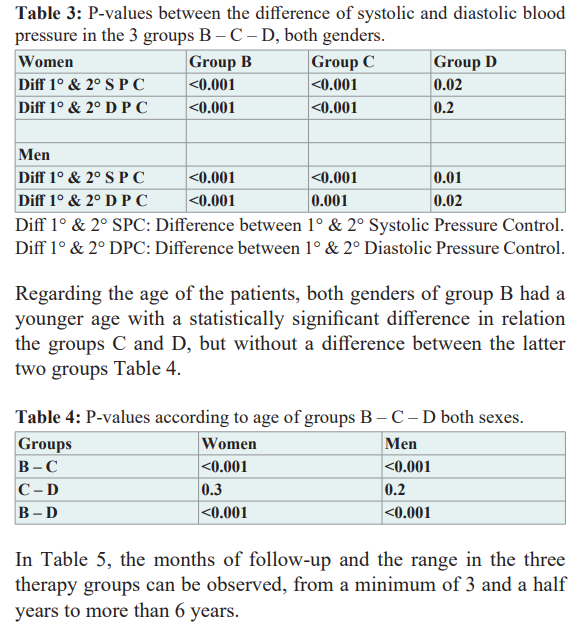
Instead, the values of the differences between the first and second measurement of systolic and diastolic pressure of the three groups, both sexes, revealed a statistically significant difference, suggesting a therapeutic benefit, except in group D of women between the first and second value of diastolic pressure Table 3.
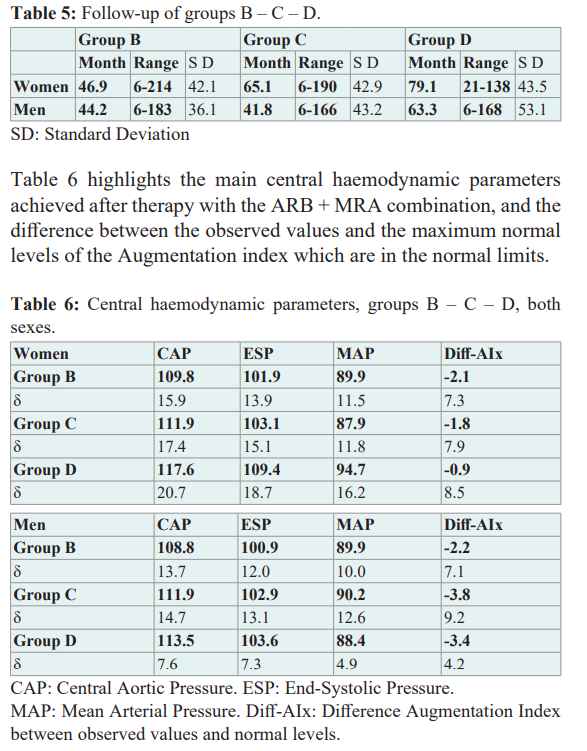
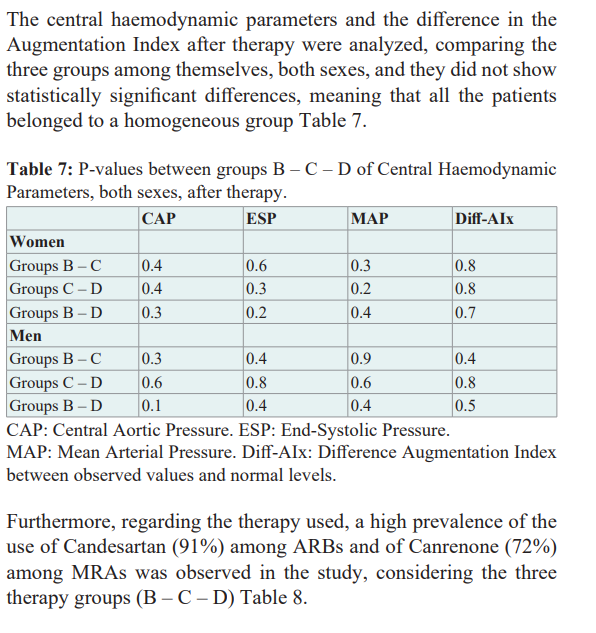
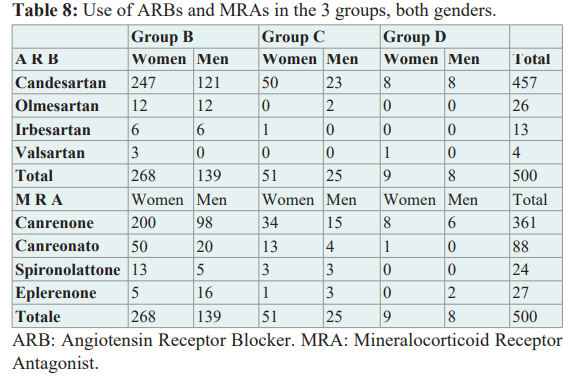
Discussion
In this sub-study, it was observed that hypertensive patients treated with the ARB + MRA combination, who previously had not or had short phases of atrial fibrillation in the 24-hour Holter monitoring (Groups B – C, respectively), did not develop atrial fibrillation in the future if the mean age at the start of therapy was up to 65 years and the mean arterial pressure reached 121/73 mmHg.
Instead, if therapy was started at an average of 68/71 years (women/men) and an average arterial pressure of 127/74 mmHg was reached, 3.4% of patients (Group D) have presented short phases of atrial fibrillation during their evolution. No patients were recorded in Group A, those who without having had data of previous atrial fibrillation, during therapy with ARB + MRA presented long episodes or short phases of atrial fibrillation.
This benefit could be attributed to the effective therapeutic association of ARB + MRA, as shown in the original study on the prevention of atrial fibrillation which postulates that the double action on Angiotensin and Aldosterone by blocking their receptors simultaneously and sequentially, for the objective to stop its harmful manifestations on the cardiovascular system, such as the onset of atrial fibrillation beyond the simple reduction of the values of the blood pressure [34].
MRAs may also be moderately effective in preventing incident or recurrent atrial fibrillation events, considering their action on vascular volume reduction and arterial stiffness, [35] decreasing wall stress, both atrial and ventricular, although they are not currently recommended as first-line drugs.
The European Society of Hypertension/European Society of Cardiology guidelines placed the MRAs as fourth-line agents to be used in resistant hypertension [36].
In fact, no guideline has ever clearly indicated when a mild decline in renal function, in a patient taking Renin Angiotensin Aldosterone System antagonists, should be accepted and not lead us to fear associated acute kidney injury.
The PATHWAY-2 study demonstrated that the steroidal MRA, spironolactone, reduced systolic blood pressure compared to other patients with normal renal function and treatment-resistant hypertension. Hyperkalemia was not a major problem in the study as patients had normal renal function [37].
The antiarrhythmic effects of MRAs may be partially explained by their impact as an established therapy for heart failure on improving cardiac mechanics, reducing left atrial pressure, and delaying disease progression [38,39].
Finally, in a larger meta-analysis of blood pressure-lowering treatment outcome studies (115 studies, 440,026 patients), the protective effect of blood pressure lowering progressively declined with significant reductions in blood pressure to a nadir of 125/75 mm Hg, and the benefit of early initiation of treatment [40].
We have been able to observe that atrial fibrillation is a consequence of a disease and not a disease in itself to be treated, considering that it could take a long time to notice its onset, from the apparently "asymptomatic" onset of the Hypertensive Cardiovascular Disease, until its registration in the electrocardiogram or as short phases in the 24-hour Holter monitoring.
Several early signs and symptoms of hypertensive cardiovascular disease have been recorded in young people and the augmentation index (surrogate value of arterial stiffness) was high when even the blood pressure values were still "normal" on the sphygmomanometer (Box), maybe it will to usefulness a more accuracy protocol for early diagnosis and not just a simple systolic/diastolic numerical threshold to the diagnosis of arterial hypertension. Also, knowing if there is a limit age and systolic and diastolic blood pressure values to be reached in their therapy to avoid the onset of atrial fibrillation.
Finally, treating the causes that could develop atrial fibrillation seems more logical, safe, effective, and accessible to a late antiarrhythmic therapy or ablation with its own procedure risks [41]. Obviously, this interpretation intends to stimulate research considering the need for confirmation with later studies.
Conclusion
It is important to accurately understand the natural history of Hypertensive Cardiovascular Disease, treat it early, and achieve the lowest systolic and diastolic blood pressure values of the current guidelines, using ARB + MRA therapy as a double sequential blockade of the RAAS, with the aim of to prevent the onset of atrial fibrillation.
Box
What does the finding of minimal mitral regurgitation mean? European Society of Hypertension & International Society of Hypertension Congress –2021. Paterno Marchioli, CA.
Is the minimal mitral regurgitation a marker of high blood pressure, and can predispose to the appearance of atrial fibrillation?
European Society of Cardiology Congress– 2021. Paterno Marchioli, CA.
What is the significance and the meaning from finding a wandering atrial rhythm? European Society of Hypertension Congress – 2022. Paterno Marchioli, CA.
The importance of the symptoms for diagnosing and treating of hypertension in children, adolescents and young-aged, assessed with applanation tonometry.
European Society of Hypertension Congress – 2022. Paterno Marchioli, CA.
Early diagnosis of hypertensive disease in youth with normal systolic and diastolic blood pressure levels.
European Society of Hypertension Congress – 2023. Paterno Marchioli, CA.
Acknowledgement
I thank all the patients who believed in my idea and do not have atrial fibrillation today.
References
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; 37: 2893-2962.
- Chen LY, Benditt DG, Alonso A. Atrial fibrillation and its association with sudden cardiac death. Circ J. 2014; 78: 2588- 2593.
- Rattanawong P, Upala S, Riangwiwat T, et al. Atrial fibrillation is associated with sudden cardiac death: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2018; 51: 91- 104.
- Bryan Williams, Giuseppe Mancia, Wilko Spiering, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018; 33: 3021-3104.
- Psaty BM, Furberg CD, Kuller LH, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001; 161: 1183-1192.
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002; 360: 1903-1913.
- Vasan RS, Larson MG, Leip EP, et al. Impact of the high- normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001; 345: 1291-1297.
- Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991; 84: 40-48.
- Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013; 34: 2746-2751.
- Koch Weser J. Nature of inotropic action of angiotensin on ventricular myocardium. Circulation Research. 1995; 16: 230- 237.
- De Mello WC. Is an intracellular renin angiotensin system involved in the control of cell communication in heart?. Journal of Cardiovascular Pharmacology. 1994; 23: 640-646.
- De Mello WC. Renin angiotensin system and cell communication in the failing heart. Hypertension. 1996; 27: 1267-1272.
- De Mello WC, Cherry R, Mannivanan S. Electrophysiologic and morphologic abnormalities in the failing heart; effect of enalapril on the electrical properties. Cardiac failure. 1997; 3: 53-62.
- De Mello WC. Cell coupling and impulse propagation in the failing heart. Journal of Cardiovascular Electrophysiology. 1999; 10: 1409-1430.
- Harada K, Sugaya T, Murakami K, et al. Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation. 1999; 100: 2093-2999.
- Horiuchi M, Ashita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999; 33: 613-621.
- Virdis A, Neves MF, Amiri F, et al. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002; 40: 504-510.
- Min LJ, Mogi M, Li JM, et al. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res. 2005; 97: 434-442.
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res. 1997; 35: 30-34.
- Horisberger JD, Rossier BC. Aldosterone regulation of gene transcription leading to control of ion transport. Hypertension. 1992; 19: 221-227.
- Weber KT. Aldosterone in congestive heart failure. New England Journal of Medicine. 2001; 345: 1689-1697.
- Barrera-Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney disease: pathophysiological basis. Kidney Int. 2019; 96: 302-319.
- Epstein M. Aldosterone and mineralocorticoid receptor signaling as determinants of cardiovascular and renal injury: from Hans Selye to the present. Am J Nephrol. 2021; 52: 209- 216.
- Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004; 66: 1-9.
- Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019; 42: 293-300.
- Benitah JP, Vassort G. Aldosterone upregulates Ca2+ current in adult rat cardiomyocytes. Circulation Research. 1999; 85: 1139-1145.
- Carmeliet E, Vereecke J. Cardiac Cellular Electrophysiology. Boston, Dordrecht/London. Kluwer Academic Publishers Group. 2002; 1-7.
- Reil JC, Hohl M, Selejan S, et al. Aldosterone promotes atrial fibrillation. Eur Heart J. 2012; 33: 2098-2108.
- Touyz RM, Schiffrin EL. Left ventricular hypertrophy in hypertension: its arrhythmogenic potential. Heart. 2005; 91: 250-256.
- Lendeckel U, Dobrev D, Goette A. Aldosterone-receptor antagonism as a potential therapeutic option for atrial fibrillation. Br J Pharmacol. 2010; 159: 1581-1583.
- Pitt B. “Escape” of aldosterone production in patients with left ventricular dysfunction treated with an angiotensin converting enzyme inhibitor: implications for therapy. Cardiovascular Drugs Therapy. 1995; 9: 145-149.
- Madu EC, Baugh DS, Gbadebo TD, et al. Effect of ethnicity and hypertension on atrial conduction: evaluation with high- resolution P-wave signal averaging. Clin Cardiol. 2001; 24: 597-602.
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension:
- Effects at different baseline and achieved blood pressure levels-overview and meta-analysis of randomized trials. J Hypertens. 2014; 32: 2150-2160.
- Paterno Marchioli CA. The double sequential actions of the Angiotensin II Receptor Blockers and Mineralocorticoid Receptor Antagonists therapy on the Renin Angiotensin Aldosterone System produce a better reduction of both blood pressure and central haemodynamic parameters and can prevent the appearance of the atrial fibrillation. Cardiol Vasc Res. 2023; 7: 1-12.
- Oraii A, Healey JS, Kowalik K, et al. Mineralocorticoid receptor antagonists and atrial fibrillation: a meta-analysis of clinical trials. Eur Heart J. 2024; 45: 756-774.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and of the European Society of Cardiology. Eur Heart J. 2013; 34: 2159-2219.
- Williams B, MacDonald TM, Morant S, et al. British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015; 386: 2059-2068.
- Tsutamoto T, Wada A, Maeda K, et al. Effects of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001; 37: 1228-1233.
- Vizzardi E, D’Aloia A, Giubbini R, et al. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. Am J Cardiol. 2010; 106: 1292-1296.
- Manta E, Thomopoulos C, Kariori M, et al. Revisiting cardiovascular benefits of blood pressure reduction in primary and secondary prevention: focus on targets and residual risk - A systematic review and meta-analysis. Hypertension. 2024; 81: 1076-1086.
- Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019; 321: 1261-1274.