Balancing Immunity with a Dietary Supplement Derived from Five Edible Plants for Management of Diabetes
Author'(s): Sirithip Wiriyachitra, Pichaet Wiriyachitra* and Ampai Panthong
Asian Phytoceuticals Public Company Limited, Bangkok, THAILAND.
*Correspondence:
Wiriyachitra P, Asian Phytoceuticals Public Company Limited, Thailand, Tel: +662 646 4882, E-mail: pw@apco.co.th
Received: 29 Jun 2024; Accepted: 13 Aug 2024; Published: 21 Aug 2024
Citation: : Sirithip Wiriyachitra, Pichaet Wiriyachitra, Ampai Panthong. Balancing Immunity with a Dietary Supplement Derived from Five Edible Plants for Management of Diabetes. Clin Immunol Res. 2024; 8(2): 1-3.
Abstract
This study investigates the immunomodulatory effects of Diabenox/BIM-D, a dietary supplement derived from
five edible plants, namely black sesame, guava fruit, mangosteen aril, pennywort leaves, and soy protein, on the
regulation of T helper cell subpopulations. Twelve healthy volunteers were divided into two groups, one receiving a
placebo and the other taking Diabenox/BIM-D capsules for 15 days. Blood samples were collected before and after
the supplementation period, and peripheral blood mononuclear cells (PBMCs) were isolated and stimulated to assess
cytokine production.
In the present study, we demonstrated that Diabenox/BIM-D can modulate the immune cell functions by reducing the
production of pro-inflammatory cytokines, including TNF-α, IL-5, IL-12 and IL-17. Diabenox/BIM-D is therefore
suggested as the food supplement for balancing immunity in the management of diabetic patients.
Keywords
Introduction
The development of specific agents affecting T helper cell subpopulations, i.e., Th1, Th3 and Th17 differentiation, has drawn special attention in the last decades. Many natural products have been reported as effective agents for balancing the immune response by regulating the differentiation of T helper cell subpopulations. These products have the potential to be immune modulators for the treatment of various diseases, including infectious diseases, cancers, autoimmune diseases and diabetes.
In this study, we aim to investigate the immunomodulatory effects of Diabenox/BIM-D, a dietary supplement consisting of five edible plants, namely black sesame, guava fruit, mangosteen aril, pennywort leaves and soy protein. We investigate the possible effect of Diabenox/BIM-D on the control of T helper cell subpopulation differentiation.
Objective
The objective of this study is to investigate the possible effects of Diabenox/BIM-D on the regulation of T helper cell subpopulations. The levels of various cytokines in blood collected from healthy donors before and after taking Diabenox/BIM-D capsules for 15 days were compared.
Study Approaches
Study Subjects
- 12 healthy volunteers: 7 males and 5 females
- Age range: 20-50 years old
- The recruited volunteers were divided into 2 groups:
- Group 1: taking a placebo; 6 subjects
- Group 2: taking Diabenox capsules; 6 subjects
Blood Collection
Blood samples (5 ml, using heparin as an anticoagulant) were collected from each subject on day 0. According to their groups, subjects took either Diabenox/BIM-D capsules or a placebo (4 capsules/day) every day for 15 days. Afterwards, blood samples (5 ml, using heparin as an anticoagulant) were collected for the second time on day 16.
Study of the Effect of the Diabenox/BIM-D Capsule on the Regulation of T Helper Cell Subpopulations
Peripheral blood mononuclear cells (PBMCs) were isolated from the collected blood using Ficoll-Hypaque gradient centrifugation. The PBMCs were then stimulated in vitro with or without anti- CD3 monoclonal antibodies (clone OKT3) and cultured for 24 hours at 37oC in a CO2 incubator. The cell culture supernatants were collected and centrifuged at 20,000 rpm for 2 minutes. The cell-free supernatants were separated and stored at -70°C for the determination of cytokines.
Determination of T helper cell cytokines
Cytokines in the cell culture supernatant were determined by FlowCytomixTM (eBioscience, Inc., San Diego, CA, USA) following the manufacturer’s protocol. Comparisons of the cytokine levels from un-stimulated PBMCs and stimulated PBMCs in terms of the Stimulation Index on day 0 and day 15 were performed.
Results
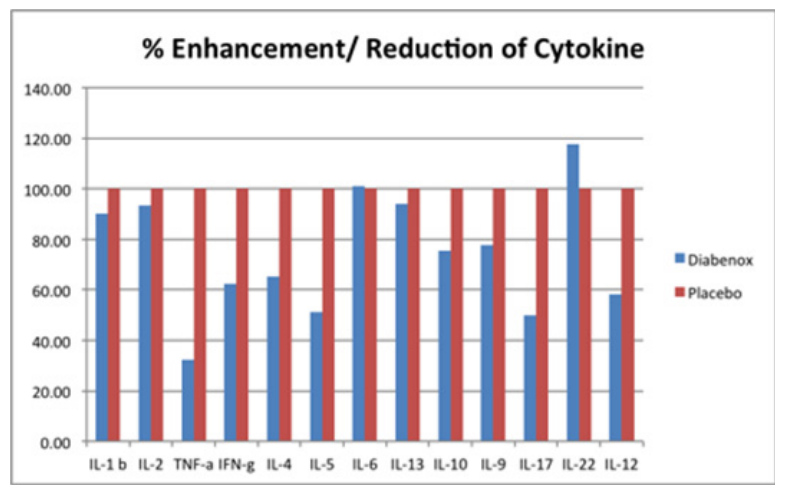
The stimulation indexes of various cytokines in subjects taking the Diabenox/BIM-D capsules and the placebo were calculated and compared. Normalization was performed using data from subjects taking the placebo. The percentage enhancement and reduction in subjects taking the Diabenox/BIM-D capsules are shown in the figure below. The results indicate that Diabenox/BIM-D capsules reduced the production of TNF-α, IFN-γ, IL-4, IL-5, IL-9, IL-10, IL-12 and IL-17.
Type 2 diabetes, which is usually associated with obesity or older age, is mostly the result of insulin resistance. In these patients, muscle or adipose cells do not respond adequately to normal levels of insulin produced by intact beta-cells. On the other hand, Type 1 diabetes usually starts in people younger than 30 and is therefore also termed juvenile-onset diabetes, even though it can occur at any age. It is a chronic autoimmune disorder that precipitates in genetically susceptible individuals due to environmental factors [1]. The body's own immune system attacks the beta-cells in the islets of Langerhans of the pancreas, destroying or damaging them sufficiently to reduce and eventually eliminate insulin production. A combination of factors, including a Th1-skewed CD4+ response as well as a deficiency of regulatory T cells, are considered important hallmarks of disease progression [2].
There is sufficient information suggesting that Th1 cells play a major role in diabetes, driving the development of disease via IFN-γ [3]. This includes observations that blockade of IFN-γ [4] or absence of STAT4 [5,6] prevents disease, whereas IL-12 promotes accelerated diabetes [7]. Tumor necrosis factor alpha (TNF-α) has well-described effects on lipid metabolism in the context of acute inflammation, such as in sepsis. Recently, increased TNF-α production has been observed in adipose tissue derived from obese rodents or human subjects, and TNF-α has been implicated as a causative factor in obesity-associated insulin resistance and the pathogenesis of type 2 diabetes. Thus, current evidence suggests that administration of exogenous TNF-α to animals can induce insulin resistance, whereas neutralization of TNF-α can improve insulin sensitivity [8]. In addition, TNF-α shows a significant positive association with insulin secretory capacity when adjusting for the effects of the confounding factors - age, sex and BMI in IGR subjects. Thus, there may be a causal relationship between TNF-α and insulin secretory defects in prediabetic and IGR subjects [9].
Th17 immunity has been demonstrated in the development of autoimmune diabetes in animal models. In NOD mice, a model of spontaneous autoimmune diabetes, inhibition of Th17 cells has been shown to regulate autoimmune diabetes [10]. IL-17 neutralization, either by anti–IL-17 or by rIL-25, prevented the development of autoimmune diabetes when given from 10 weeks of age to NOD mice [11]. Earlier treatment from 5 weeks of age did not alter diabetes progression, which suggests that Th17 immunity contributes to the progression of autoimmune diabetes during the effector phase of the disease. Natalia et al. differentiated islet- reactive BDC2.5 TcR transgenic CD4+ cells in vitro into Th17 cells and transferred them into NOD.scid and neonate NOD mice. NOD.scid recipient mice developed rapid onset of diabetes with extensive insulitic lesions, whereas in newborn NOD mice, despite extensive insulitis, most recipient mice did not develop diabetes. Surprisingly, BDC2.5+ cells recovered from diabetic NOD.scid mice, in comparison with those from neonate NOD mice, showed predominant IFN-γ over IL-17 expression, indicating conversion of donor cells into Th1 cells. Moreover, diabetes progression in NOD.scid recipients was dependent on IFN-γ while anti-IL-17 treatment reduced insulitic inflammation. These results indicate that islet-reactive Th17 cells promote pancreatic inflammation, but only induce IDDM upon conversion into IFN-γ producers [12].
In the present study, we demonstrated that Diabenox/BIM-D can modulate immune cell functions by reducing the production of pro-inflammatory cytokines, including TNF-α, IL-5, IL-12 and IL-17, which are involved in the induction of diabetes and disease progression. Reducing these cytokines may prevent the development of diabetes and improve patients’ quality of life. Diabenox/BIM-D is therefore suggested as the food supplement for balancing immunity in the management of diabetic patients.
References
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet. 2001; 358: 221-229.
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005; 23: 447-485.
- Wang B, André I, Gonzalez A, et al. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci USA. 1997; 94: 13844-13849.
- Nicoletti F, Zaccone P, Di Marco R, et al. The effects of a nonimmunogenic form of murine soluble interferon-gamma receptor on the development of autoimmune diabetes in the NOD mouse. Endocrinology. 1996; 137: 5567-5575.
- Boyton RJ, Davies S, Marden C, et al. Stat4-null non-obese diabetic mice: protection from diabetes and experimental allergic encephalomyelitis, but with concomitant epitope spread. Int Immunol. 2005; 17: 1157-1165.
- Yang Z, Chen M, Ellett JD, et al. Autoimmune diabetes is blocked in Stat4-deficient mice. J Autoimmun. 2004; 22: 191-200.
- Trembleau S, Penna G, Mortara A, et al. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J Exp Med. 1995; 181: 817-821.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000; 11: 212-217.
- Mosaraf Hossain, Omar Faruque M, Golam Kabir, et al. Association of serum TNF-α and IL-6 with insulin secretion and insulin resistance in IFG and IGT subjects in a Bangladeshi population. International Journal of Diabetes Mellitus. 2010; 2: 165-168.
- Jain R, Tartar DM, Gregg RK, et al. Innocuous IFN gamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008; 205: 207-218.
- Emamaullee JA, Davis J, Merani S, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009; 58: 1302-1311.
- Natalia MO, Chung Y, Chang SH, et al. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009; 39: 216-224.