Benefit-Risk Balance of Ketamine versus Midazolam + Fentanyl for Sedation-Analgesia in Mechanically Ventilated Patients: Randomised Controlled Trial
Author'(s):Raphaël Mubunda 1,2,3 Joseph Nsiala 1, Médard Bula-Bula 1, Berthe Barhayiga 1, Wilfrid Mbombo 1,4,Patrick Mukuna 1,2,3 Eric Amisi 1, Eric Kam Kampay5, Hugues Kwama 1,2, Patrick Makambo 1, Adolphe Mutombo 1, Marcel Kamwanga 1, Richard Mvwala 2,3, Michel Way 2, Danny Lokanga 2,Jean-Claude Mwepu3, Jamel Kimbeni3 and Degaule Ndjapanda3
1Department of Anesthesia and Intensive Care, University Clinics of Kinshasa (UCK), University of Kinshasa, DR Congo.
2Anesthesia-resuscitation department, Ngaliema Clinic, DR Congo.
3Anesthesia-resuscitation department, Clinique CHIP, DR Congo.
4Monkole hospital centre, DR Congo.
5Department of Physical Medicine and Rehabilitation, University Clinics of Kinshasa (UCK), University of Kinshasa, DR Congo.
*Correspondence:
Wilfrid Mbombo, Department of Anesthesia and Intensive Care,University Clinics of Kinshasa (UCK), University of Kinshasa,DR Congo, Tel:+243810054829.
Received: 29 Nov 2023; Accepted: 27 Dec 2023; Published: 04 Jan 2024
Citation: Mubunda R, Nsiala J, Bula-Bula M, et al. Benefit-Risk Balance of Ketamine versus Midazolam + Fentanyl for SedationAnalgesia in Mechanically Ventilated Patients: Randomised Controlled Trial. Anesth Pain Res. 2024; 8(1): 1-8.
Abstract
Objective: Ketamine has interesting pharmacological properties for sedation-analgesia in intensive care. However, there are few studies on its benefit-risk balance. The aim of this study was to evaluate the efficacy and safety of ketamine compared with midazolam + fentanyl in mechanically ventilated patients.
Methods: Randomised, non-inferiority, open-label, multicentre controlled trial. Patients aged 18 years or older requiring invasive mechanical ventilation for at least 24 hours were randomised to receive, after rapid sequence intubation, ketamine at a starting dose of 0.5 mg/kg/h (n = 191) or midazolam 0.2mg/kg/h + fentanyl 1μg/kg/h (n =191). Infusion rates were subsequently adjusted to achieve a RASS score between -2 and +1. The primary endpoint was the percentage of time spent in the RASS range -2 to +1 without the use of an alternative sedative; secondary endpoints included level of analgesia, adverse events (AEs), length of stay and mechanical ventilation, and cost of sedation.
Results: In total, 73.5% of patients in the ketamine group vs. 71.3% in the midazolam group were within the target RASS range, a difference of 2.2% [95% CI: -3.2% to 7.5%]; p = 0.18. The most frequently observed AEs in the ketamine group were hypersalivation (21.2% vs. 2.3%; p<0.001), psychodysleptic phenomena (19.8% vs. 2.6%;p<0.001) and hallucinations (9.42% vs. 1.04%; p<0.001). Delirium was the only AE more frequent in the Midazolam group than in the Ketamine group (23.5% vs 43.4%; p < 0.0001). However, the risk of arterial hypertension (7.3% vs 4.2%; p = 0.188), diarrhoea (0% vs 5%; p = 0.05) and self-extubation (3.1% vs 4.2%; p = 0.452) did not differ between the 2 groups. The length of stay in intensive care between the 2 groups was 6.3 ± 1.6 days vs 7.3 ± 1.7 days (p < 0.001) and that of mechanical ventilation 4.1 ± 0.94 days vs 4.84 ± 0.85 days (p < 0.001). The daily cost of sedative treatment was lower with ketamine than with midazolam ($32.4 ± 0.8 vs $43 ± 6.3; p<0.001).
Conclusion: In this study, the efficacy of ketamine was not inferior to that of the midazolam + fentanyl combination, but its safety was poorer. Its low cost is a real advantage in our context.
Keywords
Introduction
During a critical care unit (CCU) stay, most intubated and ventilated patients receive sedation analgesia (SA) to reduce discomfort and anxiety, relieve pain and aid ventilator adaptation [1]. Midazolam, propofol, volatile halogenated anaesthetics and, more recently, dexmedetomidine in combination with an opioid are commonly used for this purpose [2]. Despite their proven efficacy, however, these sedation and analgesia agents are not without adverse effects [3]. Midazolam, for example, because of its long elimination half-life, is responsible for delays in waking up, which are associated with longer periods of mechanical ventilation, longer stays in critical care units and increased morbidity and mortality, particularly through the acquisition of nosocomial pneumonia [4,5]. Its use is far from ideal in modern intensive care, where sedation times are becoming shorter and shorter, with attempts to wake patients up and wean them off the air every day [6]. Midazolam is also particularly prone to delirium and acute withdrawal when sedation is stopped [7].
An interesting alternative to the drugs mentioned above appears to be the use of ketamine. This compound, well known to anaesthetists since the 1960s, produces a dissociative type of anaesthesia characterised at EEG level by dissociation between the limbic and thalamo-cortical systems [8]. The patient appears awake with eyes open in slow nystagmus, but does not communicate. They are no longer connected to reality. This characteristic and its adverse neuropsychological effects (hallucination, delirium, and agitation) have limited its use in general anaesthesia in favour of other anaesthetic agents. Its use in sedation-analgesia is now increasingly common in critical care [9,10]. Unlike other agents, it produces sedation, amnesia and analgesia while preserving respiratory effort, haemodynamic stability and airway reflexes [8,11]. It is the only sedative agent to provide analgesia. It also has a bronchodilator effect comparable to that of halogen, making it the agent of choice for patients with COPD or asthma [8,11]. It also has a powerful antidepressant effect, which could be of interest to critical care patients, in particular to reduce the possible psychological consequences of hospitalisation in intensive care [12].
Once contraindicated in cases of head trauma, ketamine is no longer contraindicated [13]. More recent studies show that it may exert a neuroprotective and anticonvulsant effect by blocking the NMDA channels involved in brain damage caused by excitatory amino acids [14]. Provided that capnia is controlled by artificial ventilation and a GABAergic agent (propofol, benzodiazepine or halogen) is co-administered, ketamine reduces oxygen consumption (CMRO2), cerebral blood flow (CBF) and intracranial pressure (ICP), including in cases of intracranial hypertension [15].
A recent systematic review and meta-analysis of 15 studies, including 3 randomised studies and 12 observational studies, concluded that it is useful for sedating mechanically ventilated patients [16]. No difference in efficacy was observed between the groups treated with or without ketamine in terms of the proportion of time spent with the required level of sedation [OR 0.51 IC95% (0.14 - 1.88)]. However, the results on adverse effects were contradictory and did not allow us to establish its safety or benefit-risk balance in this indication. Hence the need for further studies to confirm or refute its safety. The aim of this study was to assess the efficacy and safety of ketamine alone compared with the combination of midazolam + fentanyl.
Methods
Study design and setting
We conducted a randomised, controlled, non-inferiority, open- label study in 2 parallel groups in the intensive care and/or resuscitation units of hospitals in the city of Kinshasa. It ran from March 2020 to March 2022. Our study was approved by the Ethics Committee of the School of Public Health of the University of Kinshasa under No. ESP/CE/011/2022. Informed consent was systematically signed by patients or their families before inclusion in the study. The authors have no conflicts of interest to declare. The study received no external funding.
Patients selection and randomisation
All consecutive patients requiring sedation-analgesia for invasive mechanical ventilation were included in this study. To be included, patients should be at least 18 years old and require invasive mechanical ventilation for at least 24 hours. Exclusion criteria were: psychiatric history, active drug addiction, severe liver failure, need for curarisation, tetanus, severe coronary artery disease, poorly controlled arterial hypertension. Randomisation in a 1:1 ratio was centralised in blocks of four. Randomisation was carried out by tossing a coin. If the coin came up heads, the next 4 patients were sedated with Ketamine and the next 4 with Midazolam + Fentanyl. If not, the order was reversed. Figure 1 summarises the sequence of our study.
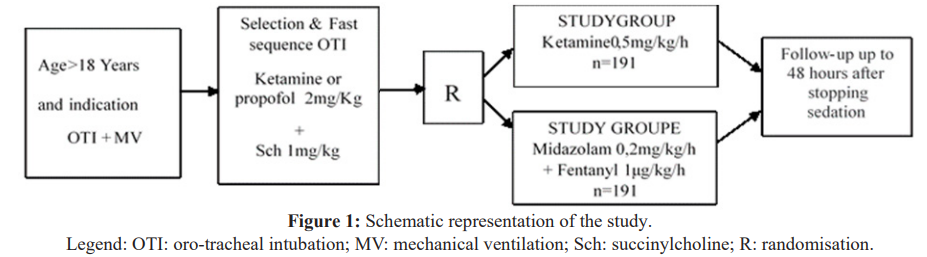
Interventions
After rapid sequence intubation, patients were randomised to receive either ketamine at a starting dose of 0.5 mg/kg/h (n = 191) or midazolam 0.2mg/kg/h + fentanyl 1 μg/kg/h (n = 191). Infusion rates were adjusted (increased or decreased by half) every 30 minutes thereafter to achieve a RASS score between -2 and +1. Patients could receive an alternative sedative agent if the desired degree of sedation was not achieved with upward adjustment of the study agent infusion. Bolus atropine 0.5 mg IVD was authorised to control hypersalivation at the discretion of the care teams. Intravenous haloperidol was authorised for the treatment of agitation or delirium in boluses of 1 to 5 mg, repeated every 10 to 20 minutes as required. Sedation could be stopped daily every morning at the discretion of the care teams to assess the possibility of permanent discontinuation of sedation. At any time during the study, the patient could withdraw from the protocol if the doctor or his or her family deemed it necessary. In both groups, the criteria for exiting the protocol were the occurrence of a side effect requiring discontinuation of the treatment, the need to use a curare, the decision of the nursing team or the decision of the patient or his family.
Data collection
Data were collected by the members of the team in charge of the patient. A research associate was responsible for checking the content and completion of the data as each patient was included. Detailed medical history, basic demographics and reason for admission to the ICU were collected at the time of study inclusion. Vital signs were recorded at least every 4 hours. Adverse events (AEs) were monitored daily until 48 hours after sedation was discontinued. These AEs were quantified using the following score: 0 = absent, 1 = minor, treatment not necessary, 2 = moderate, treatment necessary, 3 = severe, life-threatening or fatal.
Endpoints
The primary endpoint was the percentage of time spent in the target RASS range (between -2 and +1) without the use of an alternative sedative. The secondary endpoints were frequency of adverse events, total duration of mechanical ventilation and ICU stay, mortality, and average daily cost of drugs used for sedation- analgesia.
In this study, the occurrence of any noxious and unintended response inherent in the 2 study treatments was considered an adverse event. Blood pressure and heart rate were considered adverse events if systolic blood pressure was less than 90 or greater than 180 mmHg and heart rate was less than 50 bpm or greater than 120 bpm. Agitation was defined by a RASS score ≥ +2, delirium by an ICU, CAM score ≥ 3/4 during daily interruption of sedative infusion or within 48 hours of extubation and withdrawal syndrome by the presence of at least 5 of the following criteria: fever (>38°C), tachycardia (>100 bpm), hypertension (SBP > 180 mmHg), sweating, mydriasis, diarrhoea, vomiting and agitation.
Statistics
The number of subjects to be included (n) was calculated using the following formula:The non-inferiority limit (ΔL) was set at a relative difference
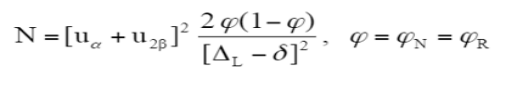
of 10% between the 2 study arms. The sample size calculation assumes that the true difference in efficacy (δ) between the 2 arms is equal to zero. The expected efficacy rate in this study was 90%. For a power of 80% (β = 20%, U2β = 0.842) and a one-sided α risk of 2.5% (uα =1.96), at least 191 patients per arm had to be included. It was decided to include 200 patients to account for study dropouts. The percentage of time spent at the required sedation level was calculated as follows: (time spent at the required sedation level / infusion time) multiplied by 100.
Definition of time spent at required level of sedation: this is the sum of time intervals (in hours) when the patient was at the required level of sedation (RASS score of -2 to +1) without the need for rescue medication. Periods when the patient was not at the required level of sedation were subtracted from this sum. Periods when the patient received rescue medication were also subtracted from this sum.
Definition of infusion duration: this is the sum of the time intervals (in hours) during which the patient received a continuous infusion of the study drug. Periods of sedation cessation were not counted in the calculation of infusion time.
All statistical analyses were performed using SPSS version 22.0 for Windows. Results are expressed as mean ± SD or percentage ± 95% CI and compared using a Mann-Whitney U test for quantitative variables or a Fisher exact test for qualitative variables. Kaplan- Meier survival curves were compared using the Log rank test. All analyses were performed on an intention-to-treat basis. No interim analysis was planned. A difference was considered statistically significant when p < 0.05.
Results
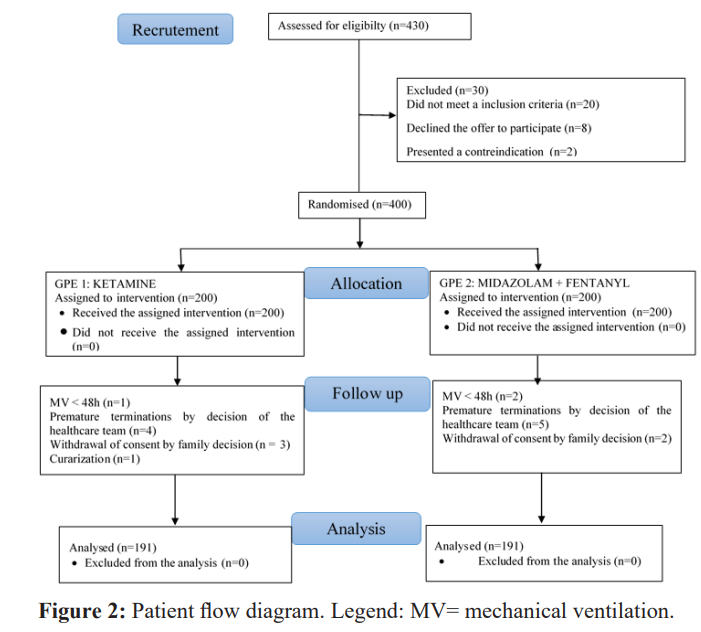
Patients flow diagram
Figure 2 show patients flow diagram of the 430 patients recruited during the study period, 30 did not meet the inclusion criteria (Figure 2). After randomisation, the 400 participants were divided into 2 groups of 200 patients each to receive either ketamine alone (group A) or the combination of midazolam + fentanyl (group B). During follow-up, 9 patients in group A were excluded (1 patient for ventilation of less than 48 hours, 4 for premature discontinuation of treatment, 3 for withdrawal of consent and 1 for need for curarization) and 9 also in group B (5 patients for premature discontinuation on the decision of the nursing team, 2 for withdrawal of consent and 2 for mechanical ventilation of less than 48 hours). In all, 191 patients were analysed in each group.
Basic characteristics of the population
The 2 groups were broadly comparable (Table 1). The mean age was 44.3 ± 10.7 years for patients in the ketamine group and 43.3± 14.1 years for those in the midazolam group, but weight was slightly higher in the control group. The 2 groups were fairly homogeneous in terms of co-morbidities, except for asthma. The reasons for admission to intensive care were similar, with the majority being medical conditions. The reasons for intubation were also identical. In both groups, coma was the main reason for intubation, followed by respiratory failure and cardiac failure.
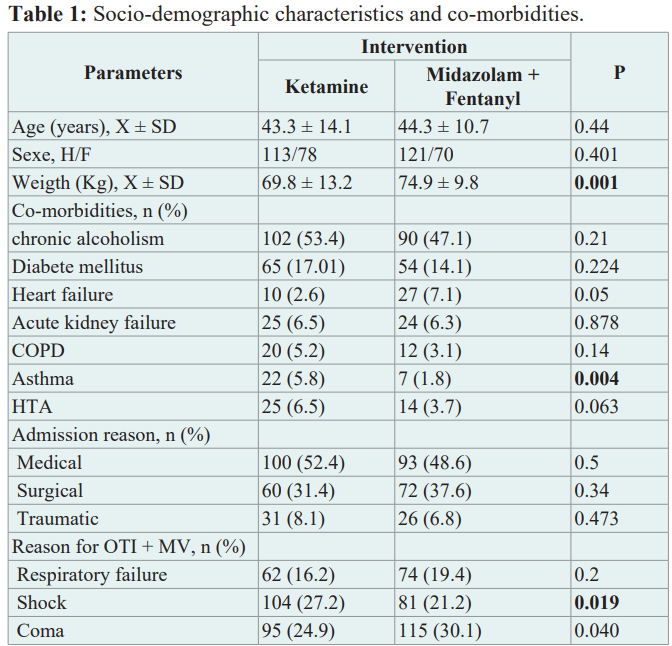
Legend: X = mean; SD = standard deviation; M = male, F = female; COPD= chronic obstructive pulmonary disease; HTA = arterial hypertension;OTI = oro-tracheal intubation; MV = mechanical ventilation; RR = respiratory failure.
Comparison of the efficacy of sedation-analgesia
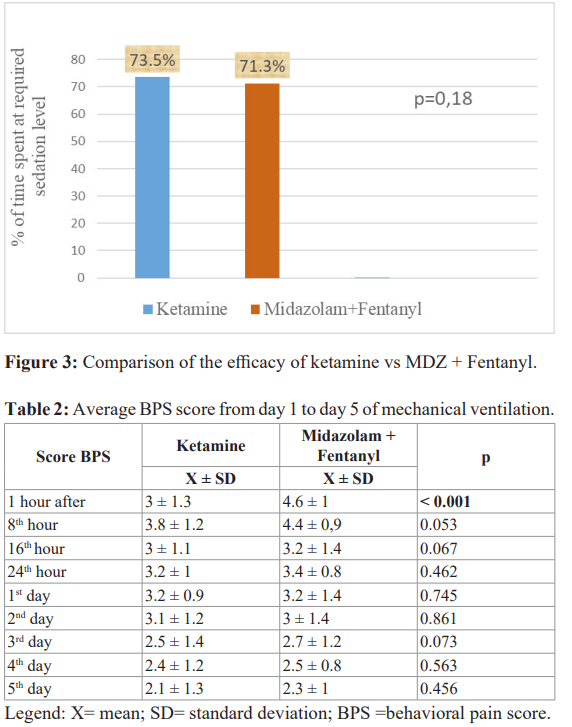
There was no difference in efficacy between the 2 treatments (figure 3). The percentage of time in the RASS target range without the use of another sedative agent was 73.5% for patients treated with ketamine versus 71.3% for patients treated with midazolam; a difference of -2.2%; 95% CI [-3.2 - 7.5%] (p = 0.18). The non- inferiority limit corresponding to a value not to be exceeded between the 2 groups was set at 10%. The null hypothesis was therefore rejected in favour of the hypothesis that ketamine was not inferior to the combination of midazolam + fentanyl in terms of efficacy. Nor was there any difference in the mean BPS scores from D1 to D5 (table 2). In addition, the use of an alternative sedative agent to maintain the depth of sedation within the target range was similar in the 2 groups (44% vs 54.9%; p = 0.56).
Comparison of tolerability and daily cost of sedative treatment
Haemodynamic tolerance during the first 48 hours
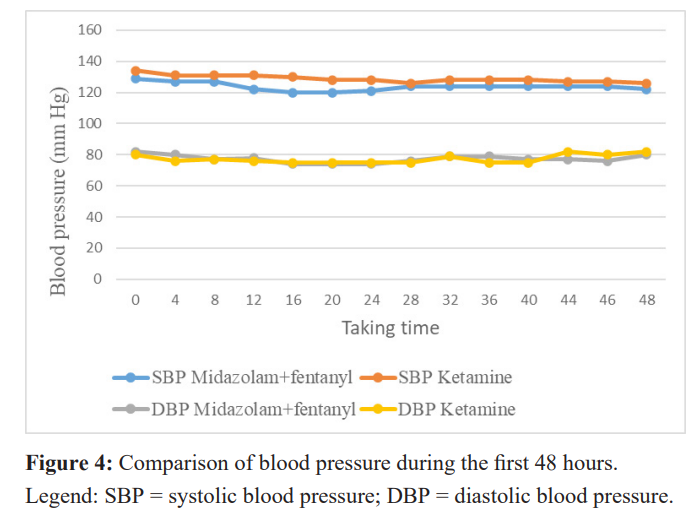
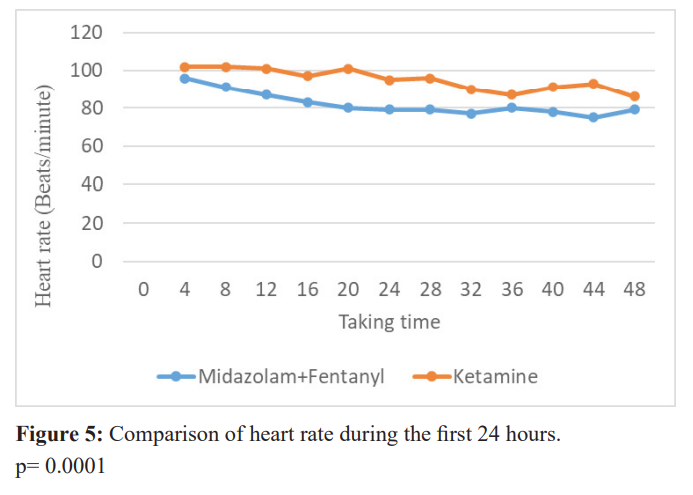
The tolerance of ketamine was not identical to that of midazolam. Figure 4 shows that the systolic blood pressure of patients in the ketamine group during the first 48 hours was always higher than that of the midazolam + fentanyl group (p≤0.05), until the second day. Diastolic blood pressure, on the other hand, showed no significant difference between the two groups (p>0.05). Similarly, the heart rate of patients in the ketamine group (figure 5) tended to be higher than that of patients in the midazolam group (p= 0.0001).
Frequency of adverse events
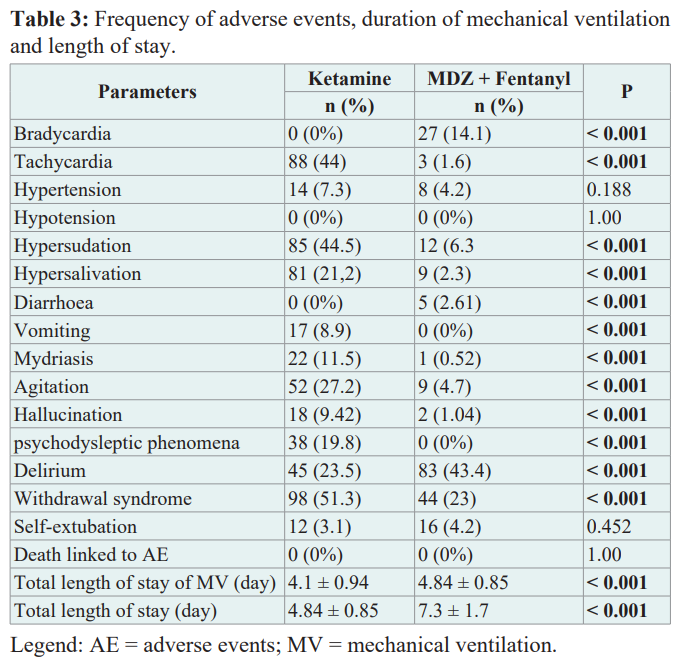
Table 3 compares the frequency of adverse events (AEs) in the 2 groups. The majority of these AEs were of minor to moderate intensity and the severity was identical in both arms. No deaths were attributed to the study treatments. The most frequently observed AEs in the ketamine group were hypersalivation (21.2% vs. 2.3%; p<0.001), psychodysleptic phenomena (19.8% vs. 2.6%; p<0.001) and hallucinations (9.42% vs. 1.04%; p<0.001). Delirium was the only AE more frequent in the Midazolam group than in the Ketamine group (23.5% vs 43.4%; p < 0.001). In contrast, the risk of arterial hypertension (7.3% vs 4.2%; p = 0.188), diarrhoea (0%vs 5%; p = 0.05) and self-extubation (3.1% vs 4.2%; p = 0.452) did not differ between the 2 groups.
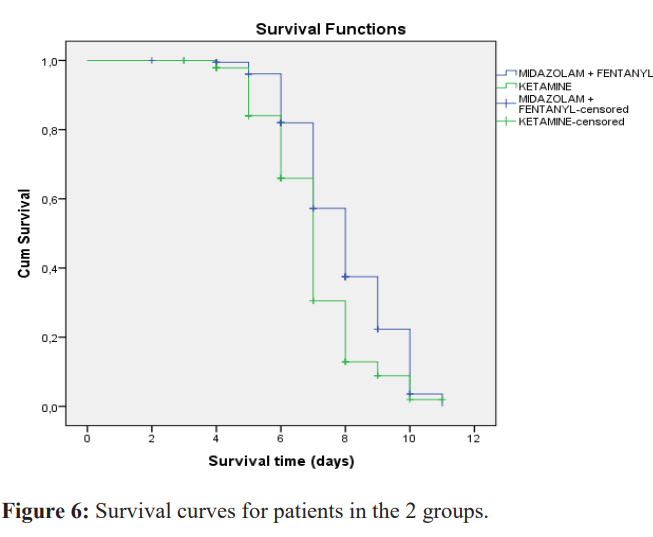
Comparison of length of stay, duration of mechanical ventilation and survival curve
Figure 5 show the comparison of heart rate during the first 24 hours.
The mean duration of mechanical ventilation was 4.1 ± 0.94 days in the ketamine group versus 4.8 ± 0.85 days in the midazolam group (p < 0.001). The mean length of stay in the ICU between the start of sedation with one of the study treatments and the end of the first ICU stay was 6.3 ± 1.6 days for patients in the ketamine group and 7.3 ± 1.7 days in the midazolam group (p < 0.001). Figure 6 shows no significant difference between the 2 survival curves. The daily cost of sedative treatment was $32.4 ± 0.8 for ketamine compared with $43 ± 6.3 for the midazolam + fentanyl combination (p = 0.001).
Discussion
The results of this study demonstrated the non-inferiority of ketamine compared with the combination of midazolam and fentanyl on the primary endpoint, i.e. the percentage of time spent in the required RASS interval (between -2 and +1) without the need for rescue medication. Over and above the sedation objectives, the study provided us with additional data on the level of analgesia, adverse effects, patient outcome and the cost of sedation treatment. The level of analgesia was comparable between the two treatment arms.
Systolic blood pressure and heart rate tended to be higher in the ketamine group. Adverse events were more frequent in the ketamine group than in the midazolam group. A reduction of one day in the length of stay in intensive care and of 0.74 days in the duration of mechanical ventilation was observed in the ketamine group, with no effect on patient survival. Finally, the daily cost of sedative treatment was $10.4 lower in favour of ketamine.
Our efficacy results are in line with the literature. Indeed, several systematic reviews and meta-analyses have confirmed the efficacy of ketamine used alone or in combination with other hypnotics for the sedation of intubated-ventilated patients [16-18]. The mechanism of action is complex. It is mainly linked to a powerful non-competitive antagonism of the glutamate N-methyl-D- aspartate (NMDA) receptor. Blocking NMDA receptors interferes with the transmission of information from peripheral areas to the brain. Ketamine also acts as an opioid receptor agonist [8,9,14]. Very recently, in an observational study, Groth et al. [19] assessed sedation-analgesia before and after the addition of ketamine by continuous infusion in 25 intensive care units in the USA between 2014 and 2017.
A statistically significant increase in the proportion of time spent within the required sedation score range was observed 24 hours before (57.1%) compared with 24 hours after (64.1%) and 48 hours after (68.9%) (p < 0.001) suggesting a positive effect of ketamine. The same was true for the pain score 24 hours before (68.9%) versus 24 hours after (78.6%) and 48 hours after (80.3) (p < 0.001). In addition, the addition of ketamine reduced the doses of opioids, midazolam, propofol and dexmedetomidine. However, contrary to our hypothesis, a higher proportion of patients in our study experienced AEs in the ketamine group than in the midazolam group. Although minor, these events have in the past been responsible for a certain reluctance to use this drug in anaesthesia. In intensive care, several descriptive studies have reported side effects associated with its use. For example, in a retrospective study by Umunna et al. [20] of ventilated intubated patients who received ketamine by continuous infusion for sedation, the incidence of adverse events was 13% (CI: 5%-30%). This study included only a small sample of patients. More recently, Pendleton et al. [21] evaluated the incidence of adverse events associated with ketamine used as an intensive care sedative in adult patients mechanically ventilated for more than 24 hours. At least one unintended effect attributed to ketamine was documented in 24% of cases.
In our study, we observed a benign increase in blood pressure and heart rate with no consequence on the need for catecholamines. This result is in agreement with the meta-analysis by Manasco AT et al. [16]. According to several authors, ketamine produces these cardiovascular effects by stimulating the central nervous system and the sympathetic system and, to a lesser extent, by inhibiting the reuptake of noradrenaline in sympathetic nerve endings [8]. It is not known whether these haemodynamic changes are detrimental or beneficial in critically ill patients. However, this meta-analysis did not demonstrate whether the use of ketamine has an effect on reducing the need for exogenous catecholamines.
Our study showed a reduction of almost 20% in delirium episodes in the ketamine group compared with the midazolam group. Although some studies suggest that benzodiazepines are particularly vulnerable to delirium [7], the literature is controversial on this subject. In the meta-analysis by Manasco AT et al. [16], 4 studies evaluated the central nervous system complications of continuous infusion ketamine for sedation analgesia during mechanical ventilation in intensive care. The incidence of ketamine-associated delirium was 38.7% compared with 52.5% without ketamine in 2 studies [22,23]. The other two studies presented the results as odds ratios. Ketamine was associated with no difference in one study [24] and an increased incidence of delirium in the other [25]. Thus, although the data currently available in the literature are heterogeneous, the trend tends to be in favour of a lower risk of delirium with ketamine.
Finally, one of the very frequent adverse effects observed in our study was hypersalivation. This result is in line with other studies. In the Pendleton et al. study cited above, sialorrhoea occurred in 6% of patients treated with ketamine, compared with no patients in the non-ketamine group. In contrast, Umunna et al. [20] showed that there was no increased hypersalivation when ketamine was infused at 2.0 mg/kg/h for analgesia and sedation. Thus, Casamento A et al. [18], in their meta-analysis of the efficacy and tolerability of ketamine during mechanical ventilation, were unable to conclude whether this potential side effect is clinically relevant when low-dose ketamine is used for sedation-analgesia in mechanically ventilated intubated adult patients. However, in our opinion, caution must be exercised. A mucus aspirator should not be far away. If necessary, it has been shown that ketamine- induced bronchial and salivary hypersecretion can be prevented by administering Atropine [26].
With regard to the impact of sedation analgesia in the ICU, the three criteria most frequently measured in the literature are duration of mechanical ventilation, length of stay in the ICU and mortality. Our study shows that ketamine reduces the mean duration of mechanical ventilation by 0.7 days and the mean length of stay in intensive care by 1 day. By way of comparison, in the meta- analysis cited above [16], three studies (n = 287) presented data on the duration of mechanical ventilation, showing no difference between the groups (mean difference 0.4 days [CI95%: 0.6 - 1.4], p= 0.47) [22,23,27]. For ICU length of stay, analysable data were reported in four studies (n=312). In contrast to our study, sedation with ketamine was associated with a longer ICU stay (mean difference 2.4 days [95% CI: 1.3 - 3.5], p<0.001) but the studies were very heterogeneous. Conversely, Robinette et al. [25] found no difference in ICU length of stay. Furthermore, the lack of difference in mortality observed in our study has been reported by several authors [22,24,27-30].
Although in most studies the reduction in the duration of mechanical ventilation and the reduction in the length of stay in intensive care between the groups with or without ketamine are too small to influence patient survival, the choice of hypnotic remains strategic. Availability and cost are clearly important factors to be taken into account in the benefit-risk balance, particularly in a context of limited resources and/or restricted healthcare expenditure. In the light of this study, although the average daily cost was in favour of ketamine, this advantage had to be balanced against a surplus of undesirable effects, which were not very serious overall, as no deaths were attributed to the treatments in the study.
Limitations
We opted for a non-inferiority study rather than a superiority study, because an increase in efficacy in the context of sedation-analgesia in intensive care is deleterious. Indeed, it has been shown that over-sedation is associated with numerous complications [4,5]. Midazolam was chosen as the reference treatment because it is the most commonly used drug for sedation during mechanical ventilation in the literature [2]. However, our study has limitations that are important to note. Firstly, the 2 groups were not completely homogeneous despite randomisation. Some patient characteristics differed between the 2 groups. Secondly, the study was conducted in a single-blind fashion. The investigator assessed the patient while being aware of the treatment received by the patient. Measurement bias cannot therefore be ruled out. However, conducting a double- blind study requires considerable logistical resources, which we did not have for this study.
Conclusion
In this study, the efficacy of ketamine in terms of maintaining depth of sedation and level of analgesia was not inferior to that of the combination of midazolam and fentanyl. In addition, the use of ketamine was associated with a reduction in the duration of mechanical ventilation and the length of stay in intensive care. On the other hand, the overall rate of adverse events was higher in the group of patients who received ketamine compared with those who received midazolam. Nevertheless, these adverse events were generally not severe and had no impact on mortality. Finally, the daily cost was significantly lower in favour of ketamine. It is therefore highly probable that ketamine has a similar efficacy to the combination of midazolam + fentanyl, and it is therefore tolerance and cost that should be discriminating factors when choosing a hypnotic agent for the sedation-analgesia of mechanically ventilated patients in intensive care. The results of our study further encourage its wider use in a context of limited resources and/or control of healthcare expenditure.
References
- Devlin JW, Skrobik Y, Gélinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the Crit Care Med. 2018; 46: 825-873.
- Payen JF, Chanques G, Mantz J, et Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007; 106: 687-695.
- Riker RR, Fraser Adverse events associated with sedatives, analgesics, and other drugs that provide patient comfort in the intensive care unit. Pharmacotherapy. 2005; 25: 8-18.
- Fraser GL, Devlin JW, Worb CP, et Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta- analysis of randomized trials. Critical Care Medicine. 2013; 41: 30-38.
- Shehabi Y, Bellomo R, Kadiman S, et al. Sedation Intensity in the first 48 hours of mechanical ventilation and 180-day mortality: a multinational prospective longitudinal cohort Crit Care Med. 2018; 46: 850-859.
- Kress JP, Pohlman AS, O Connor MF, et Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000; 342: 1471-1477.
- Ng KT, Shubash CJ, Chong JS. The effect of dexmedetomidine on delirium and agitation in patients in intensive care: systematic review and meta-analysis with trial sequential Anaesthesia. 2019; 74: 380-392.
- Peltoniemi MA, Hagelberg NM, Olkkola KT, et Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016; 55: 1059-1077.
- Hurth KP, Jaworski A, Thomas KB, et The reemergence of ketamine for treatment in critically ill adults. Crit Care Med. 2020; 48: 899-911.
- Gershengorn HB, Wunsch H. Temporal trends and variability in ketamine use for mechanically ventilated adults in the United Ann Am Thorac Soc. 2022; 19: 1534-1542.
- Nowacka A, Borczyk Ketamine applications beyond anesthesia - A literature review. Eur J Pharmacol. 2019; 860: 172547.
- Duman RS, Li N, Liu RJ, et Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012; 62: 35-41.
- Mads Christian Tofte Gregers, Søren Mikkelsen, Katrine Prier Lindvig, et al. Ketamine as an Anesthetic for Patients with Acute Brain Injury: A Systematic Review. Neurocrit Care. 2020; 33: 273- 282.
- Sleigh J, Harvey M, Voss L, et Ketamine — more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care. 2014; 4: 76-81.
- Cohen L, Athaide V, Wickham ME, et The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: systematic review. Ann Emerg Med. 2015; 65: 43-51.
- Manasco AT, Stephens RJ, Yaeger LH, et Ketamine sedation in mechanically ventilated patients: A systematic review and meta-analysis. J Crit Care. 2020; 56: 80-88.
- Miller AC, Jamin CT, Elamin EM. Continuous intravenous infusion of ketamine for maintenance Minerva Anestesiol. 2011; 77: 812-820.
- Casamento A, Niccol T. Efficacy and safety of ketamine in mechanically ventilated intensive care unit patients: a scoping Crit Care Resusc. 2023; 24: 71-82.
- Groth CM, Droege CA, Connor KA, et Multicenter Retrospective Review of Ketamine Use in the ICU. Crit Care Explor. 2022; 4: 0633.
- Umunna BP, Tekwani K, Dave Barounis D, et al. Ketamine for continuous sedation of mechanically ventilated patients. J Emerg Trauma Shock. 2015; 8: 11-15.
- Pendleton KM, Stephenson LE, Goeden N, et al. Ketamine Infusion for Sedation and Analgesia during Mechanical Ventilation in the ICU: A Multicenter Evaluation. Crit Care Res 2022; 30: 9853344.
- Shurtleff VR, Patanwala Delirium and coma-free days in patients receiving continuous-infusion ketamine versus propofol. Critical Care Medicine. 2018; 46: 462-466.
- Perbet S, Verdonk F, Godet T, et Low doses of ketaminereduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: A randomised double-blind control trial. Anaesthesia Critical Care Pain Medicine. 2018; 37: 589-595.
- Garber PC, Harger NJ, Mueller WE, et Continuous-infusion ketamine for adjunctive analgosedation in mechanically ventilated patients. Critical Care Medicine. 2018; 46: 442-446.
- Robinette E, Weant K, Hassig T, et Effects of Ketamine on Sedation And Delirium In Mechanically Ventilate Adults. Critical Care Medicine. 2018; 46: 447.
- Heinz P, Geelhoed GC, Wee C, et al. Is atropine needed with ketamine sedation? A prospective, randomised, double blind Emerg Med J. 2006; 23: 206-209.
- Bourgoin AA, Charbit M, Vialet R, et al. Safety of sedation with ketamine in severe head injury patients: Comparison with Critical Care Medicine. 2003; 31: 711-717.
- Quisilema Cadena JMCE, González Hernández. Comparison of two sedative-analgesia regimens in ventilated critical patients at the Hospital Hermanos Revista Mexicana de Anestesiologia. 2017; 40: 155-161.
- Christian Von der Brelie, Michael Seifert, Sergej Rot, et al. Sedation of Patients with Acute Aneurysmal Subar achnoid Hemorrhage with Ketamine Is Safe and Might Influence the Occurrence of Cerebral Infarctions Associated with Dela yed Cerebral World Neurosurgery. 2017; 97: 374-382.
- Jason M Reese, Victoria Fernandes Sullivan, Nathan L Boyer, et A Non-Comparative Prospective Pilot Study of Ketamine for Sedation in Adult Septic Shock. Military medicine. 2018; 183: 409-413.