Botox for Unremitting Chronic Migraine: Assessment of Muscle Function and Strength, Efficacy and Safety after More Than 10 Years of Continuous Treatment
Author'(s):Guy P Boudreau1*, Mathew Laquerre2 and Catherine Demers3
1Montreal Headache and Research Clinic, 300 Leo Pariseau,Montreal, Canada.
2University of Montreal, Doctor of Pharmacy, Director and Owner of a Pharmacy Under the Banner of Familiprix, Canada.
3Study Coordinator Specialized in Headache Medicine Research, Canada.
*Correspondence:
Guy P Boudreau, Montreal Headache and Research Clinic, 300 Leo Pariseau, Montreal, Canada.
Received: 10 Jun 2022; Accepted: 17 Jul 2022; Published: 22 Jul 2022
Citation: Boudreau GP, Laquerre M, Demers C. Botox for Unremitting Chronic Migraine: Assessment of Muscle Function and Strength,Efficacy and Safety after More Than 10 Years of Continuous Treatment. Anesth Pain Res. 2022; 6(2): 1-6.
Abstract
Objectives: Our objectives were 3-fold: Assess the impact of the injection paradigms on muscle function and strength, assess the efficacy and safety over 10 years of repeated treatments every 3 months, identify risk factors maintaining chronicity in this unremitting migraine population.
Method: One hundred patients were injected with a Botox dilution ratio of 1:1 with a sterile 0.9 % saline solution, 50 patients with a 100u vial of Botox in one, 1cc tuberculin syringe, and 50 patients with a 200u vial of Botox in two-1cc tuberculin syringe during 10 years.
Results: The strength of the paracervical and first portion of the trapezius muscle was altered in 6% (100u) and 18% (155u) of subjects. The strength of the second portion of the trapezius muscle was altered in 2% (100u) and 10% (155u) of subjects.
Muscle function of the paracervical and first portion of the trapezius muscles was altered in 34% (100u) and 28% (155) of subjects. For the second portion of the trapezius, muscle 58% (100u) and 52% (155) of subjects. The efficacy of Botox was constantly maintained during the 10 years of treatment. 72% (100u), and 74% (155u) of subjects had less than 7migraine days /month (77% improvement). Early onset of migraine, comorbid emotional burden and chronic neck pain, should be considered as risk factors for the unremitting condition.
Conclusion: Muscle strength, and function alteration did not have an impact on esthetics of the face and on normal daily muscle function in both cohorts. In both cohorts, more than 70% of patients had more than 75% improvement in monthly migraine days. Depth of the toxin injection, diffusion and presence of adipose tissue (lean versus obese patients) may be responsible for muscle strength and function alteration.
Keywords
Introduction
This study is an observational study assessing a cohort of chronic migraineurs that were very incapacitated at baseline, that have improved with continuous treatment with Onabotulinum ToxinA (Botox)
Eight percent of the migraine population evolve to very frequent monthly migraines (chronic migraine). Remission rates from chronic migraine were observed in 58.2% of patients, partial remission in 17.7%. The subjects assessed in this study, are the 24.1% that have persistent unremitting chronic migraine [1]. Farinelli published in 2006, a five-year long experience using Botox at a dose of 100 units with a fixed dose fixed site paradigm, and 12 injection sites from April 2001 to July 2006 [2].
Botox was approved by Health Canada in October 2011. The PREMPT I Trial (2006/02/23-2008/07/16) and the PREEMPT II Trial (2006/02/07-2008/08/11) were performed to assess the efficacy of Botox for the treatment of chronic migraine (155 units, 31 injection sites, fixed dose, fixed site with possible dose increase up to 200 units, every 3 months). That study demonstrated the efficacy and safety of onabotulinum toxin A (Botox) for the treatment of chronic migraine during 2 years [3]. The compel study provided additional clinical evidence for the consistency of the efficacy and for the long-term safety and tolerability of Botox for the prevention of headache in those suffering of chronic migraine who were treated with Botox every 12 weeks over 2 years (9 treatments) with the fixed dose fixed site injection paradigm from December 2011 to October 2013 [4].
Recently Rosenberg published in October 2018 the results of his study finding a sustained efficacy of the Botox treatment for migraine over 3 years [5].
To our knowledge this is a first study assessing the strength and function of injected muscles with Botox during 10 years in adults suffering of chronic unremitting migraine comparing 100 units and 155 units 0f Botox with a dilution of 1/1.
Objectives
Our objectives were 3-fold: Assess the impact of the injection paradigms on muscle function and strength, assess the efficacy and safety over 10 years of repeated treatments every 3 months, identify risk factors maintaining chronicity in this unremitting migraine population.
Rational
The dilution of the toxin, the size of the needle, the depth of the injected muscles may have an impact on the spread of the toxin on muscle function and strength and on the duration of the effect [6]. The effect of Botox may last approximately 3-4 months in motor nerves and 6-9 months in autonomic nerves.
There is a dissociation between pain and muscle relaxation. Improvement in muscle tone and pain are separate dimensions. Numerous peri cranial injections of Botox are administered to target the projections of the trigeminal nerve and cervical nerves to attenuate the input to central neurons that become sensitized and perpetuate chronic migraine.
Chronic migraine patients may have pre-existing neck pain and weakness [7]. Patients need to be assessed at baseline and during continuous treatment for muscle strength and function. The mechanisms behind the evolutive process to chronic migraine remains unknown, but genetic and epigenetic factors, inflammatory processes and central sensitization may play an important role in unremitting chronic migraine [8].
Method The Injection Paradigms
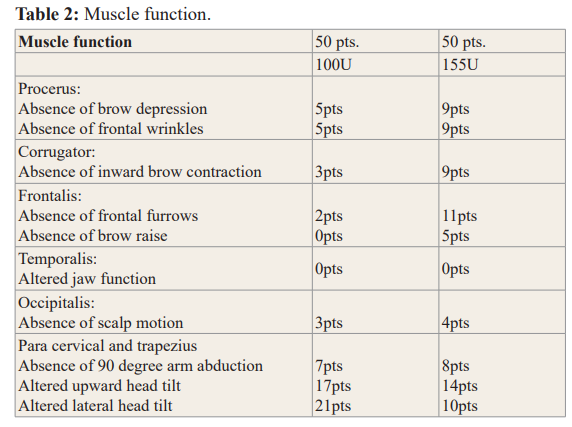
All patients were injected with a Botox dilution ratio of 1\1 with a sterile 0.9 % saline solution, in the 100u vial of Botox one, 1cc tuberculin syringe, and in the 200u vial two, 1cc tuberculin syringe, with a 30 gauge 1 inch needle were prepared with 100u, 16 sites were injected, 5 units per site; 4 sites in the frontalis muscle (20u), 2 sites in the temporalis muscle (10u), 4 sites in the paracervical muscles (20u), 6 sites in the trapezius muscle (50u).
Injection of the procerus, corrugators, occipitalis muscles were optional and episodic, depending on the need for pain relief. Trapezius doses were spared for those optional doses with 200 units, 31 sites were injected, 5 units per site; 4 Sites in the frontalis muscle (20u), 1 site in the procerus muscle (5u), 2 sites in the corrugator muscles (10u) 8 sites in the temporalis muscles (40u), 6 sites in the occipitalis muscles (30u), 4 sites in the paracervical muscles (20u), 6 sites in the trapezius muscle (30u).
From the first of March 2022 to the first of June 2022, after consenting, the first 50 patients receiving 100 units, and the first 50 patients receiving 155 units. Every 3 months for more than 10 years were assessed for muscle strength, muscle function, efficacy and safety, and risk factors for the unremitting condition.
The Muscle Function and Strength
With the 100 U modified PREEMPT paradigm, and the 155 U PREEMPT paradigm, the injected muscles were examined for alteration in the following functions and strength [9]:
- Does the procerus depress the medial end of the eyebrow, and wrinkles the skin of the glabella?
- Can the corrugator move the eyebrow down and inward?
- Can the frontalis raise the eyebrows, and modify the presence of frontal furrows from hairline, is there muscle atrophy?
- Can the occipitalis permit scalp motion?
- Can the temporalis permit prognation, retraction of the mandible, lateral jaw motion with opposed resistance, is there an hourglass deformation in the temples?
- Can the paracervical muscles and the trapezius muscle permit the extension and lateral tilt of the head measured with a goniometer?
Does the trapezius muscle permits a 90 degree arm abduction, and maintain shoulder raise with opposition.
The assessment of the strength of the paracervical muscles and trapezius was performed with the 2 cm chin trust test: characterized by the maintenance of the trust for more than 15-20 seconds, when lifting the head 2 cm. from the table while in a supine position [10,11].
Clinical examination with the TWSTRS (Toronto Western Spasmodic Torticolis Rating Scale) was performed for patients with dystonia. The search for hyperalgesia in the C2-C6 metameres was performed with the pinch and roll test.
Demographics and Risk Factors for the Unremitting Condition
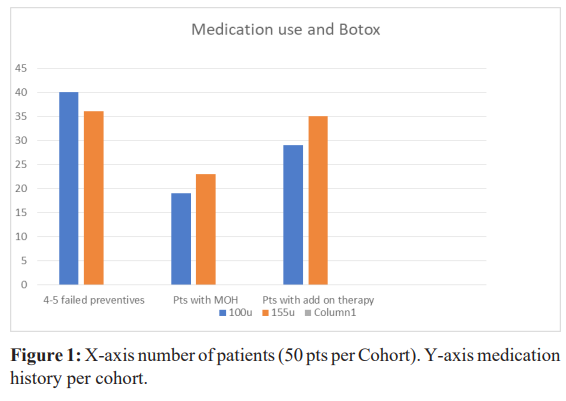
All patients were invited and consented to answer the following questions concerning: their age, their weight, the age at onset of migraine, the number of parents with migraine, the age at onset of chronic migraine.The number of preventive drugs tried before the prescription of Botox. The date of first Botox (Figure 1).
The history of depression and anxiety, physical and psychological violence and sexual abuse was registered [8].The history of stressful life events since childhood, and the presence of chronic insomnia was assessed. The history of head and neck trauma before starting Botox, and the presence of neck pain was logged.
Efficacy and Safety of Botox
The efficacy of Botox was monitored with the following questions: In the past 3 months was the frequency of your headaches 1-4 days/month, 4-7 days/month, 7-15 days/month, more than 15 days/month, have you experienced any adverse events?
We monitored the use of add on prophylactic drugs, and the weekly use of pain pills for any painful conditions?
Results
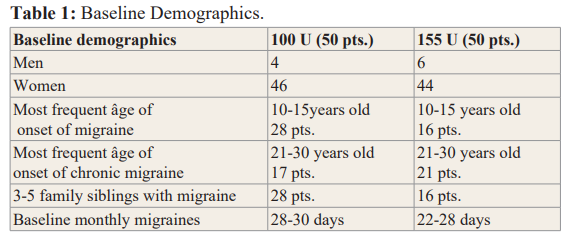
We studied 100 patients that have received Botox every 3 months for more than 10 years. Fifty patients in the 100 units cohort of Botox with a modified PREEMPT protocol. Fifty patients in the 155 units cohort of Botox with the PREEMPT protocol. For both cohorts, Botox was diluted with a 0.9% saline solution without preservatives in a 1:1 ratio.Demographics (Table 1).
In the 100u cohort, 92% (n-46) were females, in the 155u cohort. 88% (n-44) were females.
Age at assessment, in the 100u cohort, 82% (n-41) were between the age of 46-70, in the 155u cohort 72% (n-34) between the same age as above. Age at onset of migraine in the 100u cohort, 66% (n-33) of patients started migraine between the age of 10-20, in the 155u cohort, 60% (n-25) of patients started at the same age. At baseline, in the 100u cohort, monthly migraine days were 28-30 days, 96% (n-48) suffered of neck pain, 62% (n-31) had suffered of whiplash injuries. In the 155u cohort, monthly migraine days were 22-28 days, 100% (n-50) suffered of neck pain, 60% (n-30) had suffered of a whiplash injury. In the 100u cohort, 80% (n-40) and in the 155u cohort, 72% (n-36) had failed 4-5 oral migraine preventive medication.
During the 10 years of treatment with Botox every 3 months, in the 100u cohort 38% (n-19), and in the 155u cohort 46% (n-23) started overusing rescue medication for any painful conditions (neck pain, back pain, joint pain, headache, fibromyalgia) more than 2 days/ week every week. In the 100u cohort, 58% (n-29) and in the 155u cohort, 70% (n-35) added on an oral preventive for migraine or neck pain to their Botox treatment.
Muscle strength, the temporalis, the paracervical muscles and the trapezius muscle were tested for strength after 10 years of repeated treatments. There were no alterations in the temporalis muscle strength. The strength of the paracervical and first portion of the trapezius muscle was altered in the 100u cohort in 6% (n-3) of subjects, and in the 155u cohort in 18% (n-9) of subjects (Figure 2).
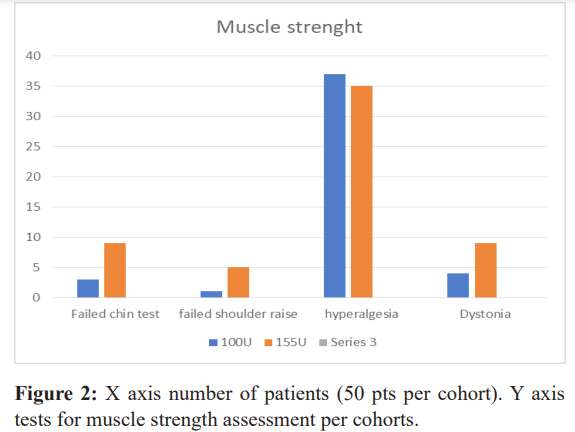
The strength of the second portion of the trapezius muscle was altered in 2% (n-1) of subjects in the 100u cohort, 10% (n-5) in the 155u cohort.
Muscle function, of the procerus, corrugator, and occipitalis, was mildly altered by repeated Botox injections, but there was no impact on the facial esthetics and no muscle atrophy.
Muscle function of the paracervical and first portion of the trapezius muscles permits upward and lateral head tilt, and the second portion of the trapezius muscle permits a 90-degree arm abduction; there was no significant impact on daily function. In the 100u cohort, for the paracervical and first portion of the trapezius muscles 34% (n-17) of subjects had function alterations and for the second portion of the trapezius muscle 58% (n-29) had function alteration. In the 155u cohort, for the paracervical and first portion of the trapezius muscles, 28% (n-14) of subjects had function alterations and for the second portion of the trapezius muscle 52% (n-26) had altered function (Table 2).
Seventy four percent (n-37) in the 100u cohort, and 70% (n-35) in the 155u cohort had hyperalgesia the day of their injections, in the C2-C6 metameres bilaterally, assessed with the pinch and roll test.
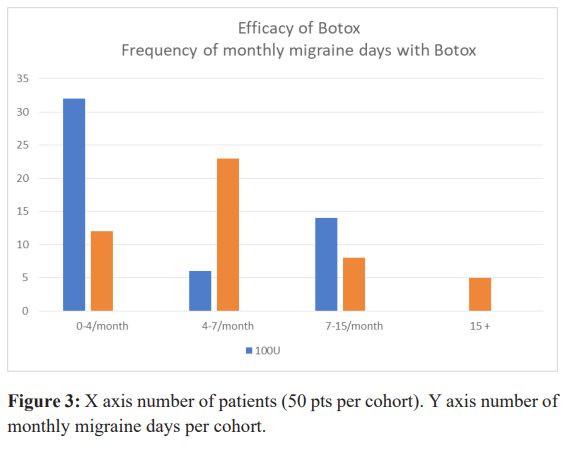
Efficacy of Botox was constantly maintained during the 10 years of treatment in this unremitting cohort. In the 100u cohort 72% (n-36), and in the 155u cohort, 74% (n-37) of subjects had a 77% (less than 7migraine days /month) improvement compared to the baseline monthly migraine frequency. In the 155u cohort 5 patients still had more than 15 migraine days /month, but had noticeable reduction in the intensity of the migraine headaches and reduced frequency of cervical pain (Figure 3).
Adverse events during the 10 years of treatment were mild and of short duration, 1 case of lid ptosis, 1 case of tearing from 1 eye, lateral elevation of the eye brow one side or both sides and easily correctable, occasional trapezius muscle spasm relievable with a muscle relaxant. No systemic adverse events were signaled during the 10 years of treatment. There was no loss of efficacy. Increasing from 100u to 155u for some patients improved their condition, for others no difference was observed and patients returned to 100u.
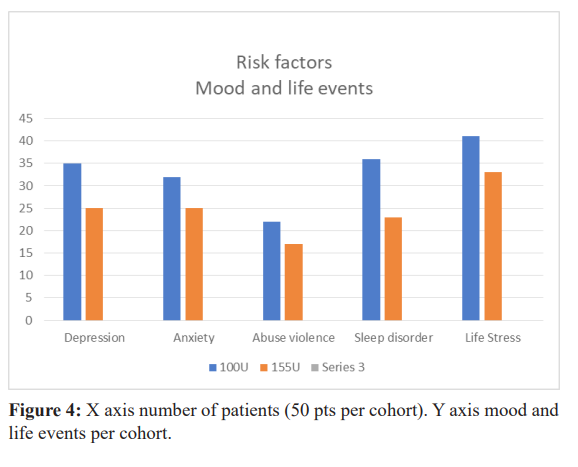
Risk factors for unremitting chronicity have not been confirmed. Many investigators have initiated protocols to assess the roll of different risk factors [8]. In this cohort of 100 subjects, we have identified in the 100u cohort that depression 70%, anxiety 64%, sleepless nights 72%, living very stressful events in childhood or adulthood 82%, being physically or psychologically or sexually abused 44%. Was very prevalent. In the 155u cohort, depression 50%, anxiety 50%, sleepless nights 44%, living stressful events 50%, victim of abuse 34% was also prevalent. Because of the lower values of depression and anxiety in the 155u cohort could we consider that 155u is efficacious in improving depression and anxiety? [12].
Discussion
Dilution: before the phase III PREEMPT trial, we were using a lower volume dilution (1:1) than the dilutions used in the PREEMPT protocol which is (1:2); we maintained the (1:1) dilution instead of the (1:2) dilution because studies that have demonstrated that injections of Botox in a lower concentration and in a higher volume resulted in a greater diffusion and a larger affected area. The spread may also be affected by muscle contraction and the presence of adipose tissue in the injected sites [6].
Muscle Strength and Muscle Function
At baseline many subjects had suffered of whiplash injury and/ or head trauma, all subjects suffered of neck pain, the head and neck injuries may have a long lasting impact on neck and shoulder strength and function [7,13,14]. The subjects in this study that had neck and/or shoulder weakness could have been because of previous injury or because of the ongoing effect of the Botox injections from the previous session.
100u versus 155u
We started using Botox for chronic migraine in 1998, worldwide; physicians became interested in the use of this toxin for the treatment of chronic daily headache and chronic migraine. Different doses and different paradigms were published by, Blumenfeld, Silberstein, Dodick, Mathew, Relja [15-18].
The 100u, fixed site fixed dose was at that time the most efficacious paradigm, and in our experience 100u in selected patients with a fixed dose, fixed site paradigm was effective and safe.
Farinelli followed 1,347 subjects with chronic daily headache for 5 years. The patients received 100u, every 3 months for 1 year, then every 6 months for 4 years. The muscles injected with 100u, with a fixed dose fixed site paradigm were the frontalis, temporalis occipitalis, trapezius and semispinalis capitis and or the splenius capitis. The first cycle accomplished a maximun of 10 headache free days per month. The maximum reduction was reached after 12 months of treatment, with patients being free of attacks 23 days per month.
Efficacy, we have demonstrated that 100u of Botox may be as effective as 155u of botox for the treatment of chronic migraine. Before the Phase III PREEMPT trial, Dodick et al., demonstrated that Botox consistently reduced headache frequency in more than 50% of subjects to less than 16 headache free days per month. Blumenfeld observed a 56% reduction in the number of headache days per month. The results of the Phase III PREEMPT trial, for the 50% responder rate at the end of the open label phase, 70% of the Botox treated patients had more than 50% reduction from baseline in frequency of headache days, and 69% had more than 50% in the monthly migraine days. The PREEMPT Phase III trial has demonstrated that the protocol is efficacious and safe. In certain situation, the 100u modified protocol should be considered for some patients as it may be as efficacious as the 155u protocol. Health economics such as cost to patients, expense of medical supply to prepare the injections need to be taken in consideration depending on the type of medicare available in the country of the patient. The safety and efficacy of Botox for chronic migraine has been demonstrated in the Phase III PREEMPT trial, the injection paradigm was established based on data from earlier clinical trial experience in the United States.
Unremitting chronic migraine and risk factors, in our cohort of patients, all patients complained of chronic cervical musculosqueletal pain at baseline. We also discovered that by asking the question for all painful bodily complaints, how many days per week were the patients using pain medication, a higher number of patients overusing medication was identified. Studies have identified that medication overuse, cervical pain, High frequency of baseline of monthly migraine days, early age of onset of migraine could be related to unremitting migraine.
Conclusion
Long-term use of Botox in adults with chronic migraine may have an impact on muscle strength, and function but does not have an impact on facial esthetics and on normal daily muscle function in both cohorts. More than 70% of patients maintained more than 77% improvement in monthly migraine days in both cohorts. No serious systemic adverse events were signaled; minor adverse events were of short duration and did not prevent patients to continue their treatment. Presence of chronic neck pain is an important baseline criterion to propose Botox for a preventive treatment of chronic migraine.
Acknowledgement
We thank the patients that have consented to participate in this observational study.
Mathew Laquerre, Pharmacist for his precious recommendations and thorough review of the present document.
Catherine Demers, study coordinator for her participation in the set up of this study and data collection.
Guy Pierre Boudreau md, principal investigator and designer of the study.
References
1.Natoli JL, A Manack, B Dean, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010; 30: 599-609.
2.Farinelli I, Coloprisco G, De Filippis S, et al. Long-term benefits of botulinum toxin type A (BOTOX) in chronic daily headache: a five-year long experience. J Headache Pain. 2006; 7: 407-412.
3.Dodick DV, Turkel CC, DeGryse MS, et al. Onabotulinum toxinA for treatment of chronic migraine: Pooled results from the double-blind placebo controlled phases of the PREEMPT clinical program. Headache. 2010; 50: 921-936.
4.Blumenfeld AM, Stark RJ, Adams AM. Long-term study of the efficacy and safety of onabotulinum Toxin A for the prevention of chronic migraine. COMPEL study. J Headache Pain. 2018; 19: 13.
5.Rosenberg J. Study finds sustained efficacy of migraine over 3 years. AJMC. 2018.
6.Jeffrey TS, Jeffrey S, Kenneth A. Effect of volume and concentration on diffusion of Botulinum toxin type A. Arch Dermatol. 2004; 140: 1351-1354.
7.Ashina S, Bendtsen L, Lyngberg AC, et al. Prevalence of neck pain in migraine and tension type headache: a population study. Cephalalgia. 2015; 35: 211-219.
8.Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. The journal of headache and pain. 2019; 20: 117.
9.Blumenfeld AM, Silberstein SD, Binder WJ, et al. Insights into the functional anatomy behind the PREEMPT injection paradigm: Guidance on achieving the optimal outcome. Headache. 2017; 57: 766-777.
10.Manuel A Domenech, Phil S Sizer, Gregory S Dedrick, et al. The deep neck flexor endurance test: Normative data scores in healthy adults. PM R. 2011; 3: 105-110.
11.Grimmer K. Measuring the endurance capacity of the cervical short flexor muscle group. Aust J Physiother. 1994; 40: 251- 254.
12.Boudreau GP, Grosberg BM, Buse D, et al. Prophylactic onabotulinum toxinA in patients with chronic migraine and comorbid depression: an open label, multicentre pilot study of efficacy and safety and effect on headache related disability, depression and anxiety. Int J Gen Med. 2015; 8: 79-86.
13.Florencio LL, Siranie de Oliviera A, Ferreira Pinheiro C, et al. Comparison of cervical muscle isometric force between migraine subgroups or migraine associated neck: a controlled study. Scientific reports. 2021; 11: 15434.
14.Blumenfeld AM, Stark RJ, Adams AM, et al. Long-term study of the efficacy and safety of onabotulinum Toxin for the prevention of chronic migraine: COMPEL study. J Headache Pain 2018; 19: 13.
15.Blumenfeld A. Botulinum toxin type A for the treatment of headache. Headache. 2004; 44: 825-830.
16.Dodick DW, Mauskop A, Elkind AH, et al. Silberstein SD BONT-A study group. Botulinum Toxin type A for the prophylaxis of chronic daily headache sub group analysis of patients not receiving other prophylactic medication: a double blind placebo-controlled study. Headache. 2005; 45: 315-324.
17.Mathew NT, Frishberg BM, Gawel M, et al. BONT-A CDH study group. Botulinum toxin type A (Botox) the prophylactic treatment of chronic daily headache: a randomized placebo controlled-control trial. Headache. 2005: 45: 293-307.
18.Boudreau GP. treatment of chronic tension type headache with botulinum toxin type A: a double blind placeb-controlled clinical tria. Cephalalgia. 2005; 25: 1101-1102.