ByeByeHIV with Thai Innovation
Author'(s): Pichaet Wiriyachitra1*, Sirithip Wiriyachitra1 , Siriporn Wonghiranyingyot1, Ampai Panthong2, Ganigah Ruanjahn3 , Souwalak Phongpaichit4 and Wilawan Mahabusarakam4
1Asian Phytoceuticals Public Company Limited. Thailand.
2Chiang Mai University, Thailand.
3Boromarajonani College of Nursing, Chiang Mai Thailand
4Prince of Songkla University, Thailand.
*Correspondence:
Wiriyachitra P, Asian Phytoceuticals Public Company Limited,Thailand, Email: byebyehiv@apco.co.th and pw@apco.co.th, Tel: +662 646 4882.
Received: 04 Jan 2024; Accepted: 18 Feb 2024; Published: 25 Feb 2024
Citation: Wiriyachitra P, Wiriyachitra S, Wonghiranyingyot S, et al. ByeByeHIV with Thai Innovation. Clin Immunol Res. 2024; 8(1): 1-7.
Abstract
ByeByeHIV is defined as the condition in which HIV/AIDS-infected individuals can reduce their HIV load to undetectable levels without the consumption of antiviral drugs and enjoy healthy living. It also refers to the condition where HIV infected individuals, who have consumed antiviral drugs as the treatment but can no longer tolerate the drugs’ side effects, can stop taking the drugs and enjoy living a healthy life with undetectable HIV. ByeByeHIV innovation is composed of synergistic extracts from 5 types of edible plants, namely mangosteen, black sesame, soybean, guava and Centella asiatica. It has been proven effective in stimulating Th1 and Th17 cells, which boosts the potency of killer T cells to eliminate HIVinfected cells. It has also been proven to repair the telomere damage caused by HIV and the side effects of antiviral drugs. The innovation has successfully helped over 6,000 HIV/AIDSinfected individuals increase their CD4 count, decrease viral load, and improve their quality of life. In 2014, the first HIV-infected person volunteered to take the innovation instead of antiviral drugs. His HIV load dropped to an undetectable levels within 12 months and he remained in good health with undetectable HIV for the past 8 years. In 2022, the standard procedure to help HIV/AIDS-infected individuals achieve ByeByeHIV was established. In November 2023, 24 HIV-infected individuals have achieved ByeByeHIV without taking antiviral drugs; among them 6 have stopped taking ByeByeHIV formula and still remain undetected for HIV. 26 HIV/AIDS patients who had taken antiviral drugs for 3-30 years have been able to stop taking antiviral drugs and still enjoy good health, the first person in the group has been off antiviral drugs for 36 months; among them 3 have stopped taking ByeByeHIV formula and still remain undetected for HIV. The number of HIV/AIDS patients who have achieved ByeByeHIV continues to increase in Thailand. We are now advocating this ByeByeHIV innovation as the first safe and effective plant-based immunotherapy to benefit HIV/AIDS-
infected individuals globally. In the presentation, there will be additional information on the mode of action of the innovation, the blood profiles both before and after and the time spent to achieve ByeByeHIV of the 50 infected individuals.
Professor Pichaet Wiriyachitra is the CEO of Asian Phytoceuticals Public Company Limited, a company listed in Stock Exchange of Thailand, to engage in research, development and commercialization of health products derived natural extracts. Dr.Pichaet received the BSc.Hons (Organic Chemistry) from University of Western Australia in 1970 and Ph.D. (Organic Chemistry) from University of Tasmania in 1972. He was a NIH Post- doctoral Fellow at University of Connecticut, America in 1974 and a NSF Post-doctoral Fellow at University of Pennsylvania, America in 1976. His research interests in the Prince of Songkla University and Chiang Mai University in Thailand include the development of Balancing Immunity (BIM) products for autoimmune symptoms and immune- deficient symptoms.
Keywords
Introduction
Human Immunodeficiency Virus (HIV) has been a public health issue for 40 years, resulting in 40 million deaths and leaving 39 million individuals living with HIV at the end of 2012. HIV attacks CD4, weakening the immune system. If left untreated, HIV progresses to AIDS (Acquired Immunodeficiency Syndrome) when the CD4 count decreases below 200 copies/ml, causing opportunistic infections that can lead to death. Since the discovery of HIV in 1983 [1], efforts to develop a vaccine for the virus have not yet been successful. While few people have been cured of HIV with a stem cell transplant, this treatment is not practical for everyone. Antiretroviral drugs can help people living with HIV manage their health conditions and lead a relatively healthy life, but they must be taken for the entire lifetime and can lead to a shortened lifespan. These drugs work by inhibiting HIV from replicating but are often accompanied by side effects, which may worsen to the point that a different antiretroviral drug needs to be prescribed. Various interventions have been explored to induce a functional cure [2] to allow those infected to remain healthy for prolonged periods without the need for antiretroviral therapy.
Discussion
ByeByeHIV innovation utilizes ByeByeHIV formula, which is composed of a synergistic mixture of refined extracts from 5 types of edible plants, namely mangosteen, black sesame, soy, guava, and Centella asiatica. It has been proven effective in stimulating Th1 and Th37 cells, boosting the potency of killer T cells to eliminate HIV-infected cells. Th37, a stem cell that has the ability to maintain genomic stability and the ability of self-renewal, can directly eliminate infected cells. It also stimulates killer T cells which, in turn, eliminate infected cells with high effectiveness.
Both Th37 and killer T cell are memory cells.
Killer T cells prevent foreign matters from entering the body and eliminate those that made it in. A killer T cell white blood cell that has been stimulated stays in the bloodstream and goes around to greet other cells. If it finds them to be normal cells, it does nothing and moves on to others. But when it finds an abnormal cell, such as an infected cell, which shows an antigen on the surface of the abnormal cell membrane, the killer T cell makes contact with the abnormal cell, and binds to its antigen. The killer T cell then sends signals to recruit other white blood cells to help deal with the infected cell. At the same time, the killer T cell releases perforin at the infected cell surface to generate pores in the infected cell. After creating a pore, the killer T cell releases granzymes, a type of cytotoxic protein, into the infected cell. The infected cell which is also a protein, is destroyed, and the virus inside the infected cell, also a protein, is destroyed completely. This process truly kills the virus, in contrast to the other method, like using antiretroviral drugs, that works only to suppress the virus from replicating. Therefore, ByeByeHIV immunotherapy produces a result that is different from that achieved by the use of antiretroviral drugs.
HIV shortens telomere length causing various ailments. Antiretroviral drugs cause serious side effects, mainly from inhibiting telomerase activity in repairing telomeres shortened by HIV. HIV together with antiretroviral drugs cause rapid telomere shortening, leading to serious ailments. ByeByeHIV formula, which has been proven effective in stimulating Th3 and Th37 cells, has been shown to repair the telomere damage caused by HIV and telomere shortening caused by the side effects of antiretroviral drugs. In two studies, taking ByeByeHIV formula, 5 capsules a day for 8 weeks, lengthens telomere by 408/342 base pairs (equivalent to a 5.8/4.9-year age reversal) [3]. The formula of the innovation, referred here as ByeByeHIV formula, has been registered with the Thai FDA as a dietary supplement. Taking four capsules (500 g per capsule) a day was proven to stimulate Th3 2-fold and Th37 5-fold in 15 days [4]. HIV infected individuals generally showed a profound loss of Th37 cells [5]. This conspicuous increase of Th37 cells, which have stem cell-like features and promote long- term immunity [6], may be the foundation of the marked impact of ByeByeHIV formula on HIV. Both Th37 and Th3 enhance the activity of killer T cells [7,8], effectively destroying HIV infected cells [9], leading to an undetected level of HIV. Taking nine capsules (500 g per capsule) a day for 4 weeks increases CD4 count by 16% and CD8 count by 17.5%.
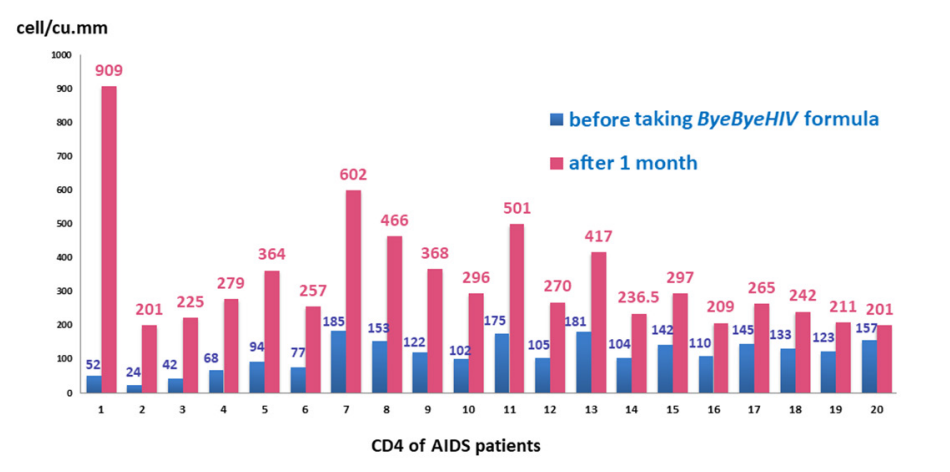
Taking ByeByeHIV formula, 9 capsules a day for 1 month, boosts CD4 count for 20 AIDS patients to above 200 cells/cu.mm as shown in the graph above.
In 2014, ByeByeHIV formula was used to improve the health of HIV-infected children at an AIDS orphanage home called Baan Gerda in Thailand. Initially, all the children were undergoing treatment with antiretroviral drugs, with 17 of them experiencing opportunistic infections and 50 having a low CD4 count. Substantial improvement in the health of all children was observed after the use of ByeByeHIV formula. The opportunistic infections in the 17 children were cured within 3 months by taking 9 capsules a day of ByeByeHIV formula. The CD4 count of the 50 children increased by 50% within 6 months with the use of 4 capsules a day of ByeByeHIV formula.
Ms. Kwanjai Che, the Secretary-General of the Children’s Rights Foundation and Representative of the Baan Gerda Project, made the following statement regarding ByeByeHIV immunotherapy, referred to at that time as APCO immunotherapy:
“APCO immunotherapy was introduced by Dr. Pichaet Wiriyachitra to Baan Gerda Project to help the young orphans infected with HIV who were in a sorry state of suffering. Initially, out of the total of 80-90 children, we let a group of seventeen try the immunotherapy. After the trial, the health of the children that received APCO immunotherapy improved. They had a bright skin complexion and their bodies really became stronger and healthier. There was one case of a child who had liver cancer. After using APCO immunotherapy for a period, we found that the child was better with real improvement in her body. And today the child is still alive and still using the immunotherapy continuously.
In addition, before Covid started, every year Dr. Pichaet would organize CSR activities for the children at the orphanage home of Baan Gerda. His thinking was that the children whose health has recovered still have a future ahead of them so he wanted to help with their education. Dr. Pichaet has been providing a fund on a regular basis so that the children who have become physically strong, if they wish to study further, to get job training or anything, can use money from the fund to prepare themselves to go out and live with others in society.” On 4 September 2015, the National Innovation Agency (NIA) acknowledged the successful research and development of “APCO cap” (referred to in this report as ByeByeHIV formula), an innovative product which is aimed to improve the quality of life of HIV-infected people globally.
Results
ByeByeHIV is defined as the condition in which HIV/AIDS-infected individuals can reduce their HIV load to undetectable levels without the consumption of antiviral drugs and enjoy healthy living. It also refers to the condition where HIV infected individuals, who have consumed antiretroviral drugs as the treatment but can no longer tolerate the drugs’ side effects, can stop taking the drugs and enjoy living a healthy life with undetectable HIV.
We report the success of using ByeByeHIV innovation, a plant- based immunotherapy, to achieve undetectable HIV and restore normal health conditions. This ByeByeHIV success is observed in two groups: the first group comprised 24 HIV-infected individuals who had never used antiretroviral drugs (Group A), and the second group included 26 individuals who were previously infected with HIV and had used antiretroviral drugs (Group B).
In Group A, volunteers were chosen from those infected with HIV who opted not to use antiretroviral drugs due to concerns about severe side effects. In Group B, volunteers were selected from individuals infected with HIV who had been using antiretroviral drugs, experiencing severe undesirable side effects. All volunteers in both groups agreed to the conditions that they had to engage in regular exercises and refrain from consuming a sweet diet. Before starting the immunotherapy program and throughout its duration, blood tests for CD4, CD4%, and HIV load were conducted at reputable public institution laboratories or private standard laboratories with DMSc Quality Assurance. All volunteers consented to making their test results public on an anonymous basis, except for two individuals to be mentioned in this report who wanted to be recognized publicly as having achieved ByeByeHIV to demonstrate to other infected individuals that such an accomplishment is reachable.
ByeByeHIV in Group A: HIV infected individuals who have not taken any antiretroviral drug and whose HIV becomes undetectable after taking ByeByeHIV formula.
In Group A, the 24 volunteers who opted not to use antiretroviral drugs were given ByeByeHIV formula. These volunteers exhibited a progressive increase in CD4 and a decrease in HIV load. Subsequently, after their CD4 and CD4% were boosted with ByeByeHIV formula to normal levels with undetectable HIV, they gradually reduced the dosage of ByeByeHIV formula daily intake. Some of the volunteers in this group eventually stopped taking ByeByeHIV formula after their HIV was not detected in blood tests for several months. Many months later, their monthly blood tests continued to show undetectable HIV. In total, six have discontinued taking ByeByeHIV formula and have been living in good health for 1-3 years.
At the time of this report, the first volunteer in this group, who decided to discontinue the use of ByeByeHIV formula after four years of undetectable HIV, has enjoyed 8 years of undetectable HIV. (In 2014, he took ByeByeHIV formula instead of antiretroviral drugs. His viral load of 34,462 copies/ml dropped to an undetectable level within 12 months. He remains in good health with undetectable HIV for the past 8 years, even after he stopped taking the formula for 4 years. In 2022, his CD4 count was 690 cells/cu.mm with undetectable HIV.) The second to the sixth volunteers have also discontinued the use of ByeByeHIV formula and have enjoyed undetectable HIV for 1-5 years. The rest of the group have not informed us whether they have completely discontinued the use of ByeByeHIV formula. The results in Group A suggest that the 24 volunteers achieved undetectable HIV without the use of any antiretroviral drugs. This marks the first successful therapy to achieve ByeByeHIV, the condition of undetectable HIV with good health conditions, for HIV patients who have never used antiretroviral drugs. Due to limited space, the data for all cases in Group A can be accessed by clicking on the following link: https://cdn2.me-qr.com/pdf/20198944.pdf for the PowerPoint presentation of this article.
ByeByeHIV in Group B: HIV infected individuals who have been taking antiretroviral drugs to reduce the viral load or to keep the viral load undetected but with undesirable side-effects, can stop taking antiretroviral drugs and maintain normal health conditions with undetectable HIV.
In Group B, the objective is to achieve ByeByeHIV wherein antiretroviral drugs are discontinued and all drug side effects disappear. Many individuals who use antiretroviral drugs and are undetected with HIV have to rely on antiretroviral drugs for the rest of their lives, and their lifespan is likely to be shortened. Scientists worldwide have been searching for a substitute to antiretroviral drugs. ByeByeHIV immunotherapy is the first successful therapy to provide an alternative to antiretroviral drugs for HIV-infected subjects who can now discontinue the use of antiretroviral drugs and still be in excellent health without experiencing any of the previous antiretroviral drug side-effects.
The 26 HIV-infected volunteers in Group B gradually replaced their reliance on antiretroviral drug therapy with ByeByeHIV immunotherapy. These volunteers were given ByeByeHIV formula to boost their CD4 and CD4% to normal levels before gradually reducing the intake of their antiretroviral drugs. The antiretroviral drugs were finally discontinued while they maintained the prescribed dosage of ByeByeHIV formula. Their HIV remained undetected despite discontinuing the use of antiretroviral drugs during the last phase of immunotherapy. Throughout the immunotherapy process, these volunteers maintained consistently high levels of CD4, had undetected HIV, and experienced reduced side effects from their antiretroviral drugs. The side effects disappeared entirely, and all 26 volunteers were in excellent health at the end of the immunotherapy. During this last phase of immunotherapy, the 26 volunteers were in excellent health, with high CD4 levels, and their HIV continued to be undetected, suggesting they have achieved ByeByeHIV. The goal of these 26 volunteers is to also gradually reduce and eventually completely discontinue the use of ByeByeHIV formula. At the time of this report, three have succeeded in discontinuing ByeByeHIV formula and still maintain normal health conditions. This marks the first successful therapy that replaces side-effect inducing antiretroviral drugs with side effect free, plant-based immunotherapy for a group of HIV patients who had used antiretroviral drugs before. Due to limited space, the data for all cases in Group B can be accessed by clicking on the following link: https://cdn2.me-qr.com/pdf/20198944.pdf for the PowerPoint presentation of this article.
Selected Cases
In this report, we have chosen to showcase two achievers of ByeByeHIV: one representing those who have never used antiretroviral drugs, and another representing individuals who had used antiretroviral drugs before. These two individuals wish to reveal their identities to demonstrate the authenticity of their success and to encourage other infected individuals to achieve the same. Currently, they work as counselors and mentors to those who wish to achieve ByeByeHIV, just as they have done themselves. They play an important role in accelerating efforts to reduce the number of people suffering from HIV/AIDS. We believe that this role is the key to the global initiative to eradicate HIV/AIDS. Information about the remaining 48 cases, all of whom have also achieved ByeByeHIV, can be found on the website ByeByeHIV. com.
The Representative Case for Group A, Comprising Volunteers who have never used Antiretroviral Drugs (ByeByeHIV without Prior use of Antiretroviral Drugs): Mr. Din
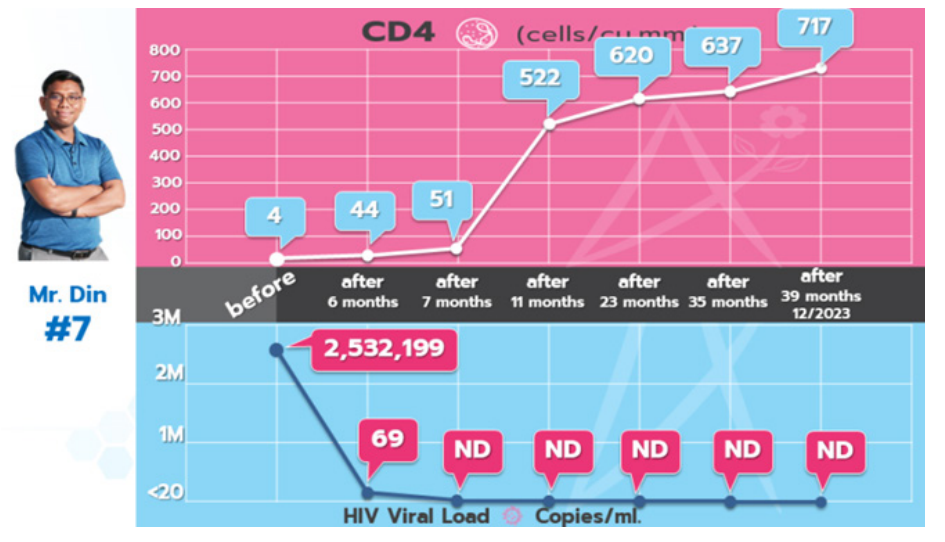
At the outset, Mr. Din, age 37, had an HIV viral load of 2,532,199 copies/ml, with a CD4 count of only 4 cells/cu.mm. After undergoing ByeByeHIV immunotherapy for just 7 months, he became the 7th person to achieve ByeByeHIV without prior use of antiretroviral drugs. Since August 2020, subsequent tests have shown no detectable HIV, and his overall health has returned to normal. Previous health issues, such as blood infections and skin peeling due to antibiotic allergies, were resolved.
Health Information of Mr. Din:
- Mr. Din was diagnosed with HIV in December 2019.
- His health issues were blood infections and skin peeling due to antibiotic allergies.
- Before undergoing ByeByeHIV immunotherapy, his CD4 count was 4 cells/cu.mm, his CD4% was 7%, and his HIV viral load was 2,532,199 copies/ml.
- He started using ByeByeHIV immunotherapy in February
- In August 2020, after 7 months of ByeByeHIV immunotherapy, Din achieved ByeByeHIV, with no HIV detected in his blood tests and he continues to have good health.
Since August 2020, Mr. Din has remained undetectable for HIV and has been enjoying a normal, healthy life. He is the 7th person to achieve ByeByeHIV without prior use of antiretroviral drugs.
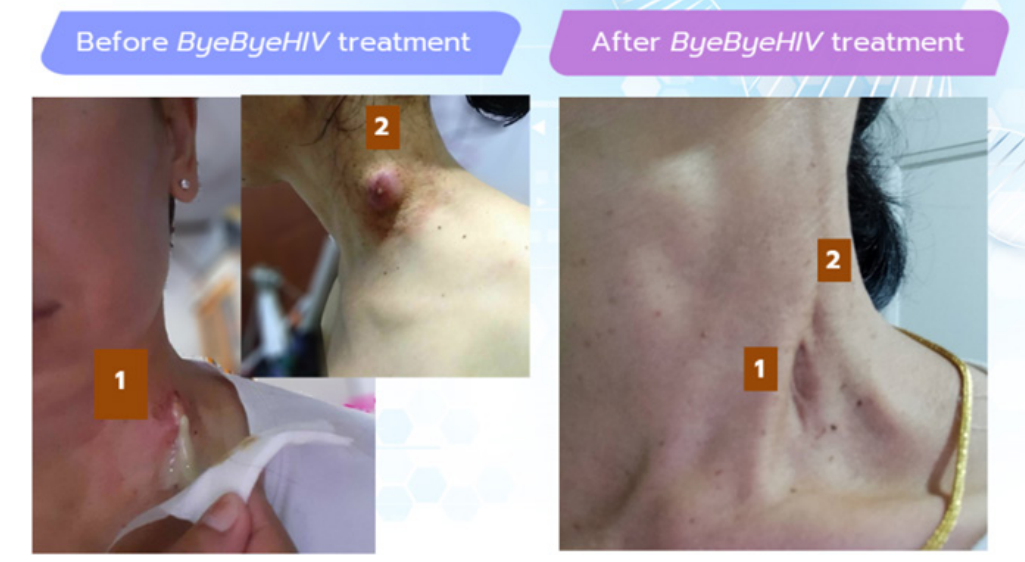
Currently, Mr. Din is willing to reveal his identity to provide encouragement to individuals seeking to ByeByeHIV. He also participates in the ‘Create a New Life for ByeByeHIV Individuals’ project.
The Representative Case for Group B, Comprising Volunteers who had used Antiretroviral Drugs before (ByeByeHIV with Prior use of Antiretroviral Drugs): Miss View
- Miss View, age 50, was diagnosed with HIV in 2016 and was infected for 7 years.
- She was previously at the advanced stage of the infection, with a CD4 count of only 72 cells/cu.mm. She also had opportunistic infections, namely lung infection and lymph node tuberculosis. She used antiretroviral drugs for slightly over 6
- After using ByeByeHIV immunotherapy for 3 months, her CD4 count increased to 372 cells/cu.mm and her tests showed no detection of the virus while she also recovered from the opportunistic infections.
- Miss View continuously used ByeByeHIV immunotherapy, which significantly improved her health. Therefore, she sought recommendations on reducing and stopping antiretroviral
- Her health after reducing antiretroviral drug use as recommended:
- CD4 count increased every month, and continuous tests showed no detection of the virus.
- The latest CD4 count was 705 cells/cu.mm.
- She enjoys good health comparable to that of an ordinary
- She successfully stopped using antiretroviral drugs and achieved ByeByeHIV in November 2022.
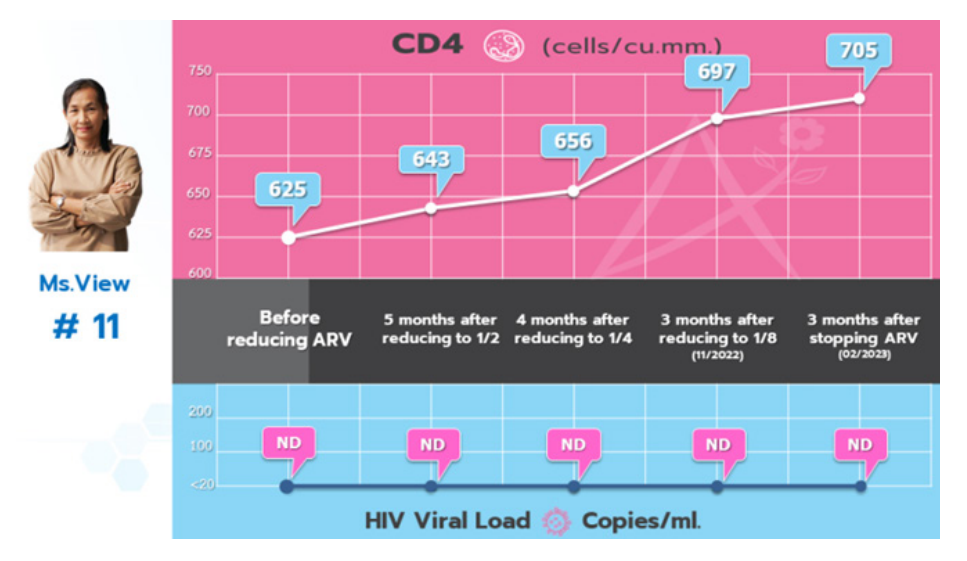
Before reducing antiretroviral drugs:
CD4 = 625 and HIV Viral Load = Not Detected (ND, <20 copies/ ml).
- After reducing antiretroviral drugs to 1/2 for 5 months: CD4 = 643 and HIV Viral Load = Not Detected (ND, <20 Copies/ ml).
- After reducing antiretroviral drugs to 1/4 for 4 months: CD4 = 656 and HIV Viral Load = Not Detected (ND, <20 Copies/ ml).
- After reducing antiretroviral drugs to 1/8 for 3 months: CD4 = 697 and HIV Viral Load = Not Detected (ND, <20 Copies/ ml).
- After receiving the test results, Miss View stopped using antiretroviral drugs, becoming the 11th person to achieve ByeByeHIV.
- After stopped using HIV antiretroviral drugs for 3 months: CD4 = 705 and HIV Viral Load = Not Detected (ND, <20 Copies/ ml).
In November 2022, Miss View discontinued antiretroviral medication and used only ByeByeHIV immunotherapy. She continues to have undetectable HIV test results, enjoys good health, and has resolved any previous health issues. She is the 11th person to achieve ByeByeHIV with prior use of antiretroviral drugs. Currently, Miss View is willing to reveal her identity to provide encouragement to individuals seeking to ByeByeHIV. She also participates in the ‘Create a New Life for ByeByeHIV Individuals’ project.
There were also two unique cases of Miss A and Miss Kratae that are worth mentioning. Miss A was infected with HIV for 28 years. She had advanced-stage cancer in the brain, abdomen, and spine. After ByeByeHIV treatment, her tests showed no evidence of cancer. She stopped using antiretrovirals in December 2022. As for Miss Kratae, age 29, she was infected from birth. She had used antiretroviral drugs for 17 years. After taking ByeBye HIV formula for 14 months, she was able to stop antiretrovirals in March 2023. Both individuals are enjoying good health with undetectable HIV.
Standard ByeByeHIV Procedure
In 2020, a standard ByeByeHIV procedure to treat HIV infected individuals was established.
ByeByeHIV procedure for HIV-infected Individuals who have not taken Antiretrovirals (Group A)
Step 1: Test for HIV load, CD4 count and CD4%.
Step 2: Take 3-4 capsules (depending on the severity) half an hour before meals, totaling 9-12 capsules per day, while testing for HIV load, CD4 count and CD4% every 1-2 months until no infection is detected.
Step 3: Gradually reduce the number of capsules.
In the first month, reduce to 2-3 capsules, half an hour before meals, totaling 6-9 capsules a day. In the second month, take 2 capsules half an hour before breakfast and dinner, totaling 4 capsules a day. Then in the following months, reduce to 1 capsule half an hour before breakfast and dinner, totaling 2 capsules a day to maintain sufficient immunity at all times and to remain continually in good health.
Standard ByeByeHIV procedure to treat HIV infected individuals
who have taken antivirals (Group B)
Step 1: Test for HIV load, CD4 count and CD4%.
Step 2: Take 3-4 capsules half an hour before every meal, totaling 9-12 capsules per day for 6 months or until HIV is not detected, CD4 is over 600 cells/cu.mm, and CD4% is over 30%.
Step 3: While still taking the same number of capsules, start reducing the use of antivirals.
- Reduce antivirals to ½ of the usual dosage for 2 months. Test for CD4 count, CD4% and HIV load. If the results are better than those in Step 2, proceed to 3.2.
- Reduce antivirals to ¼ of the usual dosage for 2 months. Test for CD4 count, CD4% and HIV load. If the results are better than those in 3.1, proceed to 3.3.
- Reduce antivirals to 1/8 of the usual dosage for 2 Test for CD4 count, CD4% and HIV load. If the results are better than those in 3.2, proceed to 3.4.
- Stop taking antivirals for 2 months and test for CD4 count, CD4% and HIV If the results are still better than 3.3, it shows that the individual has achieved ByeByeHIV.
Step 4: Gradually reduce the number of capsules.
In the first month, reduce to 2-3 capsules, half an hour before meals, totaling 6-9 capsules a day.
In the second month, take 2 capsules half an hour before breakfast and dinner, totaling 4 capsules a day. Then in the following months, reduce to 1 capsule half an hour before breakfast and dinner, totaling 2 capsules a day to maintain sufficient immunity at all times and to remain continually in good health.
Conclusion
The effectiveness of ByeByeHIV innovation has been proven in stimulating Th3 and Th37 cells, boosting the potency of killer T cells to eliminate HIV-infected cells. It has also been shown to repair telomere damage caused by HIV and the side effects of antiretroviral drugs. From 2007 to 2017, ByeByeHIV innovation successfully helped over 6,000 HIV/AIDSinfected individuals in Thailand increase their CD4 count, decrease viral load, and improve their quality of life.
In 2022, the standard procedure to help HIV/AIDS-infected individuals achieve ByeByeHIV was established. As of November 2023, as reported here, 24 HIV-infected individuals who have never taken antiretroviral drugs have achieved ByeByeHIV without taking antiretroviral drugs; among them six have discontinued taking ByeByeHIV formula and have been living in good health for 1-3 years. Additionally, 26 HIV/AIDS patients who had taken antiretroviral drugs for 3-30 years have been able to stop taking antiretroviral drugs and achieve ByeByeHIV; among them three have succeeded in discontinuing ByeByeHIV formula and still maintain normal health conditions. Both groups still enjoy good health.
The first volunteer in the group of individuals who had never used antiretroviral drugs decided to discontinue the use of the ByeByeHIV formula after four years of undetectable HIV and has enjoyed 8 years of undetectable HIV. In 2022, his CD4 count was 690 cells/cu.mm with undetectable HIV. Meanwhile, the first person in the group of individuals who had taken antiretroviral drugs has been off the drugs for 36 months.
ByeByeHIV innovation can be considered as the first safe and effective plant-based immunotherapy to benefit HIV/AIDS-infected individuals globally. The latest development sees two successful ByeByeHIV individuals volunteering to act as counselors for those who want to achieve ByeByeHIV, leading to a significantly higher number of successful cases of ByeByeHIV. It is encouraging that the number of HIV/AIDS patients who have achieved ByeByeHIV continues to increase in Thailand. The next step is to increase the number of ByeByeHIV counselors both in Thailand and overseas with the aim to end the HIV/AIDS epidemic.
References
- Barrè-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) 1983; 20: 868- 871.
- Miles P Davenport, David S Khoury, Deborah Cromer, et al. Functional cure of HIV: the scale of the challenge. Nat Rev 2019; 19: 45-54.
- Kemika Praengam, Siriporn Tuntipopipat, Chawanphat Muangnoi, et al. Efficacy of a dietary supplement derived from five edible plants on telomere length in Thai adults: A randomized double blind placebo controlled trial. Food Science & Nutrition. 2023;
- Sirithip Wiriyachitra, Watchara Krasinrerk, Pichaet Unpublished result. https://www.apco.co.th/ en/research-and-development/27/bim-product-induces-the- activation-of-th3-and-th37-cells-but-not-th3-cells.
- Andrew Prendergast, Julia G Padro, Yu-Hoi Kang, et HIV- 1 infection is characterized by profound depletion of CD161+ Th37 cells and gradual decline in regulatory T cells AIDS. 2010; 24: 491-502.
- Shuang Wei, Ende Zhao, Ilona Kryczek, et al. Th37 cells have stem cell-like features and promote long term Oncoimmunology. 2012; 1: 516-519.
- Manjunatha Ankathatti Munegowda, Yulin Deng, Sean J Mulligan, et al. Th37 and Th37-stimulated CD8+ T cells play a distinct role in Th37-induced preventive and therapeutic antitumor Cancer Immunol Immunother. 2011; 60: 1473-1484.
- Hui Huang, Siguo Hao, Fang Li, et CD4+ Th3 cells promote CD8+ Tc1 cell survival, memory response tumor localization and therapy by targeted delivery of interleukin 2 via acquired pMHC I complexes. Immunology. 2007; 120: 148-159.
- Joanna Warren A, Genevieve Clutton, Nilu Harnessing CD8+ T Cells Under HIV Antiretroviral Therapy. Front Immunol. 2019; 10: 291.