Cancer Healthcare Utilization Impact of Precision Therapeutics: Hospitalization/Emergency Visits
Author'(s): Stephanie Wonnell Yencho1, Jared Austin2, Eric Betka2, Derek Gyori3, Christine Cassidy4, Laura Stanbery5,Phylicia Aaron5, Charles Brunicardi6, Gerald Edelman4, Lance Dworkin4 and John Nemunaitis5*
1Ohio State University Wexner Medical Center, Columbus, Ohio,United States.
2Department of Pharmacy, University of Toledo Medical Center,Toledo, Ohio, United States.
3Department of Pharmacy and Pharmaceutical Sciences,University of Toledo, Toledo, Ohio, United States.
4Department of Medicine, University of Toledo, College and Life Sciences, Toledo, Ohio, United States.
5Gradalis, Inc, Carrollton, Texas, United States.
6SUNY Downstate Health Sciences University, Brooklyn, NewYork, United States.
*Correspondence:
John Nemunaitis, MD, Gradalis, Inc, Carrollton, Texas, United States.
Received: 07 July 2020; Accepted: 01 August 2020
Citation: Yencho SW, Austin J, Betka E, et al. Cancer Healthcare Utilization Impact of Precision Therapeutics: Hospitalization/Emergency Visits. Cancer Sci Res. 2020; 3(3): 1-3.
Abstract
Recent advances in comprehensive genomic profiling (CGP) has enabled the detection of actionable mutations with corresponding molecular targeted therapy, both rapidly and accurately. While the cost of CGP has declined, it is still unknown how CGP through use of targeted precision therapy effects health care costs. We present evidence for use of CGP in advanced cancer management and corresponding precision therapy to reduce total healthcare utilization.
Keywords
Introduction
Precision therapy is rapidly expanding standard of care for advanced cancer in patients with a relevant molecular signal or immune profile [1]. This particularly may affect later stage cancer patients who have exhausted standard of care options [2]. Routine use of CGP is now standard of care for patients with advanced cancer. Use of CGP companion diagnostics have demonstrated statistically significant benefit in overall survival, relapse free survival and progression free survival involving precision therapy [3-11].
Moreover, fewer toxic deaths [12], cost effective health management [3-5,11,13] and better health outcomes have been suggested [3].
Recent retrospective analysis of nearly 30,000 advanced cancer patients undergoing CGP testing and receiving target directed precision therapy via FoundationOne (Foundation Medicine, Boston USA) assessment revealed 7.2 month overall survival advantage compared to patients who did not undergo CGP testing and/or received non-targeted systemic treatment [16]. Similarly, immune response directed precision therapy mostly related to use of checkpoint inhibitors provided overall survival advantage of 8.5 months [14].
Results
Given advantage of CGP testing to guide optimal cancer treatment, we expanded our use of CGP testing towards routine assessment with FoundationOne CDx for new therapy consideration of advanced cancer patients on January 1, 2018. We compiled objective healthcare utilization evidence retrospectively including days of hospitalization and emergency room visits from January 1, 2017 to December 31, 2017 prior to routine molecular profiling and compared to January 1, 2018 through August 15, 2018 time period after establishing use of molecular profiling with FoundationOne CDx testing in similar advanced late stage cancer patients. Retrospective analysis was done using consecutive patient medical records following site Institutional Review Board (IRB) approval.
A total of 218 consecutive adult (≥ age 18) patients with advanced cancer who received systemic therapy had records reviewed; 97 patients in 2017 and 86 in 2018 (35 patient records were excluded from analysis for incomplete emergency visit and/ or hospitalization information). No difference in demographics such as age, sex, race, comorbidities (congestive heart failure, hypertension, diabetes, COPD, or chronic kidney disease), type of cancer (stage) or performance level was observed. Nineteen out of 97 (20%) of patients received precision therapy related to CGP testing in 2017 and 26 of 86 (30%) received precision therapy in 2018 (3 patients received combination precision therapy and chemotherapy and were included in the precision therapy group).
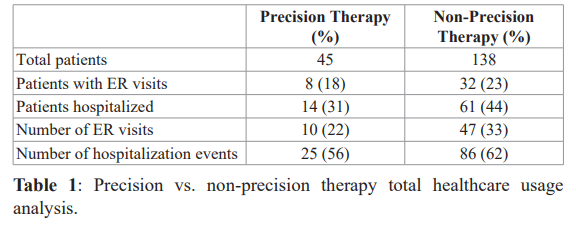
Review of precision therapy group analysis vs. non-precision therapy from January 1, 2017 to August 15, 2018 suggested a reduction in the average number of days hospitalized per hospital stay per patient admitted in the precision therapy group compared to non-precision therapy treated patients (3.36 (1-19) days vs. 4.7 (1-34) days, respectively). Furthermore, toxic events accessed as emergency room visits and acute hospitalization events, identified as “total healthcare usage” revealed 35 events occurred in the 45 precision therapy patients and 133 events occurred in the 138 patients in the non-precision therapy treatment group. Fisher exact analysis supported this as a significant difference (p=0.0004). Breakdown of total healthcare usage parameters are shown in Table 1.
Discussion
These results serve as proof of principal support and justify further studies and large population analysis of healthcare utilization impact with respect to CGP testing to guide precision therapy decisions. Routine use of CGP testing, which is necessary for therapeutic guidance, can be compared to healthcare utilization involving breast cancer screening with mammography in terms of relationships to clinical benefit and cost. Screening with mammography of women between ages 50-69 every 2 years, has been shown to prevent one breast cancer death for every 5-9 women screened [15,16]. Cost to payer is $11.40 per client per year [17]. Whereas, CGP screening of advanced cancer patients with non-small cell lung cancer done one time with corresponding utilization of precision therapy in management revealed similar prevention of death for 1 out of 5 per CGP tested patients. Cost to payer was considerably less than mammography at $0.20 per client per year [18].
A proportion of patients at our cancer center were also able to expand treatment options based on CGP guidance of precision therapy. Reitsma et al. [19] suggested further savings involved with experimental trial opportunity utilizing CGP signal guidance and precision drug cost transferred to sponsor. Although verification of activity to the experimental precision therapy is not validated. Haslem et al. [4 ] published a retrospective analysis of precision therapy outcomes in community managed cancer patients without other standard of care options and showed correlated progression free survival (PFS) improvement (22.9 weeks vs. 12 weeks p < 0.002). This approach would not be recommended in patients with early stage disease or for those with an NCCN guideline directed therapy option. However, those patients with relevant targeted clinical trial and/or palliative management options would be a consideration. Over the next 5 years, given lower toxicity profile of precision therapeutics, use will likely be expanded to enable earlier stage of disease treatment. Signorovitch [6] et al. and Chawla [3] et al. found CGP testing as cost effective when performed early in advanced cancer diagnosis.
Conclusion
In conclusion, extensive cancer patient clinical benefit has been demonstrated in numerous trials involving CGP testing directed precision therapeutics leading to FDA registration of over 100 novel precision therapeutics when based on comparison to standard non- systemic therapy. We believe these results add to accumulating evidence that routine use of CGP for cancer care management and corresponding precision therapy will reduce healthcare cost.
Acknowledgement
The authors would like to acknowledge Christina Egan for her role in facilitating the collaboration among authors.
References
- Pao W, Hutchinson Chipping away at the lung cancer genome. Nat Med. 2012; 18: 349-351.
- Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal Ann Oncol. 2019; 30: 1096-1103.
- Anita Chawla FJ, Jennifer J. Wheler, Vincent A. Miller, et al. James Signorovitch Estimated Cost of Anticancer Therapy Directed by Comprehensive Genomic Profiling in a Single-Center Study. JCO Precision Oncology. 2018.
- Haslem DS, Van Norman SB, Fulde G, al. A Retrospective Analysis of Precision Medicine Outcomes in Patients With Advanced Cancer Reveals Improved Progression-Free Survival Without Increased Health Care Costs. J Oncol Pract. 2017; 13: e108-e119.
- Signorovitch J, Janku F, Wheler JJ, et al. Estimated cost of anticancer therapy directed by comprehensive genomic profiling CGP in a single-center study. Journal of Clinical 2017.
- Radovich M, Kiel PJ, Nance SM, et al. Clinical benefit of a precision medicine based approach for guiding treatment of refractory Oncotarget. 2016; 7: 56491-56500.
- Jardim DL, Schwaederle M, Wei C, et Impact of a Biomarker-Based Strategy on Oncology Drug Development A Meta-analysis of Clinical Trials Leading to FDA Approval. J Natl Cancer Inst. 2015; 107.
- Schwaederle M, Zhao M, Lee JJ, et Association of Biomarker-Based Treatment Strategies With Response Rates and Progression-Free Survival in Refractory Malignant Neoplasms A Meta-analysis. JAMA Oncol. 2016; 2: 1452- 1459.
- Dhir M, Choudry HA, Holtzman MP, et Impact of genomic profiling on the treatment and outcomes of patients with advanced gastrointestinal malignancies. Cancer Med. 2017; 6: 195-206.
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup IFCT. Lancet. 2016; 387: 1415-1426.
- Handorf EA, McElligott S, Vachani A, et al. Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncol Pract. 2012; 8: 267-274.
- Schwaederle M, Zhao M, Lee JJ, et al. Impact of Precision Medicine in Diverse Cancers A Meta-Analysis of Phase II Clinical J Clin Oncol. 2015; 33: 3817-3825.
- Nathan A, Pennell AM, Zheng-Yi Zhou, et Economic impact of next generation sequencing vs sequential single- gene testing modalities to detect genomic alterations in metastatic non-small cell lung cancer using a decision analytic model. Journal of Clinical Oncology. 2018.
- Singal G, Miller PG, Agarwala V, et al. Association of Patient Characteristics and Tumor Genomics With Clinical Outcomes Among Patients With Non-Small Cell Lung Cancer Using a Clinicogenomic JAMA. 2019; 321: 1391-1399.
- Hendrick RE, Helvie MA. Mammography screening a new estimate of number needed to screen to prevent one breast cancer AJR Am J Roentgenol. 2012; 198: 723-728.
- O'Donoghue C, Eklund M, Ozanne EM, et al. Aggregate cost of mammography screening in the United States comparison of current practice and advocated Ann Intern Med. 2014; 160: 145.
- Villanti AC, Jiang Y, Abrams DB, et A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013; 8: e71379.
- Signorovitch J, Zhou Z, Ryan J, et al. Budget impact analysis of comprehensive genomic profiling in patients with advanced non-small cell lung J Med Econ. 2019; 22: 140-150.
- Reitsma M, Fox J, Borre PV, et al. Effect of a Collaboration Between a Health Plan Oncology Practice and Comprehensive Genomic Profiling Company from the Payer Perspective. J Manag Care Spec 2019; 25: 601-611.