Cardiac Rhythm Implications of Extracellular Magnesium
Author'(s): Katholi Richard E* and Ervin Marcella R
Prairie Educational and Research Cooperative, Department of Pharmacology, Southern Illinois School of Medicine Springfield, IL USA.
*Correspondence:
Richard E. Katholi, M.D., Prairie Cardiovascular Consultants, Ltd., P.O. Box 19420, Springfield, IL 62794-9420, Telephone:217-788-0706,
Received: 29 Nov 2022; Accepted: 04 Jan 2023; Published: 10 Jan 2023
Citation: Katholi Richard E, Ervin Marcella R. Cardiac Rhythm Implications of Extracellular Magnesium. Cardiol Vasc Res. 2023;7(1): 1-9.
Abstract
Although magnesium is mainly an intracellular cation, magnesium in the extracellular space is an important mechanism by which this cation exerts its effects on the electrical system of the heart. This is an important concept for two reasons: 1) a serum magnesium level reflects extracellular magnesium and is a sensitive indicator to determine whether magnesium is affecting cardiac rhythms, and 2) because rapid changes in serum magnesium may occur in clinical situations independent of total body magnesium resulting in important arrhythmias. Hypomagnesemia depolarizes heart cells resulting in both supraventricular and ventricular tachyarrhythmias. Keeping serum magnesium levels greater than 2 milliequivalents per liter is recommended. In contrast, hypermagnesemia hyperpolarizes heart cells suppressing atrial and ventricular arrhythmias.
Keywords
Foreword
A challenge when reviewing the literature on extracellular magnesium and cardiac rhythms is that serum magnesium can be reported as millimoles per liter (mmol/L), milliequivalents per liter (mEq/L) or milligrams per deciliter (mg/dL). When comparing articles and determining clinically significant levels, we chose to standardize by converting to mEq/L. For readers who are used to other reported measurements, mg/dL multiplied by 0.411 converts serum magnesium to mmol/L; mmol/L divided by 0.5 converts serum magnesium to mEq/L; mmol/L multiplied by 2.43 converts serum magnesium to mg/dL. When reporting therapy with intravenous magnesium sulfate (MgSO 4 ), we thought it helpful to give both grams of MgSO4 and milliequivalents of elemental magnesium (1 gram MgSO4 equals 98.6 mg of elemental magnesium equals 8.12 mEq of elemental magnesium equals 4.06 mmol of elemental magnesium).
Introduction
Magnesium is the most common mineral deficiency affecting about one-half of the United States population [1]. Magnesium is mainly an intracellular cation; extracellular magnesium represents only 1% of total body magnesium and is primarily found in serum and red blood cells. Only 0.3% of the magnesium in the body is found in the blood serum. Serum magnesium levels do not correlate well with total body stores [1,2]. However, magnesium in the extracellular space is an important mechanism by which this cation exerts its effects on the electrical system of the heart [3-5]. This is an important concept for two reasons: 1) a serum magnesium level reflects extracellular magnesium and is a sensitive indicator to determine whether magnesium is affecting cardiac rhythms, and 2) because rapid changes in serum magnesium may occur in clinical situations independent of total body magnesium resulting in important arrhythmias. The objective of this article is to review the mechanisms by which hypomagnesemia and hypermagnesemia influence cardiac rhythms in adult patients and focus on specific clinical indications for urgent intravenous MgSO4 replacement.
Effects of hypomagnesemia on the electrical system of the heart
There are two distinctly different populations of cells within the cardiac conduction system. There are the pacemaker cells of the sinus node which generate impulses (similar cells are found in the atrioventricular node) and Purkinje cells, which are abundant in the false tendons of the left ventricle and normally do not initiate impulses but conduct propagated impulses rapidly. Using microelectrodes, selective cardiac tissues (canine sinus node and false tendon) were isolated (thus, no nerve influences) and studied in an environment in which the perfusate magnesium concentration could be precisely controlled [3]. Rapid changes in extracellular magnesium concentration could be made easily, and the direct effects of either an increase or decrease in magnesium could be examined for each cell type. Removal of magnesium from the perfusate into the sinus node led to an increase of 36% in sinus rate. The sinus rate was reversed by tetrodotoxin suggesting that hypomagnesemia results in rapid sodium channel inward current as the cause of increased rate. False tendon cells responded to magnesium-free solution with depolarization followed by spontaneous activity [3,4]. Both sinus node and false tendon cells returned to normal resting transmembrane potential by restoring magnesium to the perfusing solution. These results indicate that extracellular magnesium has a direct effect on the transmembrane electrical processes. If one could extrapolate from these results, one would expect hypomagnesemia which facilitates depolarization would result in the development of sinus tachycardia as well as atrial and ventricular tachyarrhythmias.
Effects of hypermagnesemia on the electrical system of the heart
Using microelectrodes, canine sinus nodes and false tendons have also been used to study the electrical response to hypermagnesemia [3,5]. Doubling magnesium concentration slowed sinus rate by 19%. Sinus rate returned to control as soon as magnesium concentration was returned to normal. Elevation of magnesium to 4.0 mEq/L had no significant effect on false tendon cells. However, elevation of magnesium to 8.0 mEq/L caused hyperpolarization of the resting transmembrane potential. All effects of elevated magnesium on sinus node and false tendons subsided when returning to normal at 2.0 mEq/L magnesium concentration. If one could extrapolate from these results, one would expect hypermagnesemia, which facilitates hyperpolarization to promote sinus bradycardia and attenuate atrial and ventricular automatic activity, thus stabilizing electrical activity of the heart.
Effects of hypokalemia and hyperkalemia on the electrical system of the heart
Serum magnesium is held at a relatively fixed level in intact biologic systems, but it is well known that hypomagnesemia is considered arrhythmogenic, especially when associated with hypokalemia or digitalis toxicity. While hospital laboratories report "normal" serum magnesium range as 1.5-2.2 mEq/L, microelectrode measurements demonstrate that cardiac cells begin depolarizing when serum magnesium is lower than 2.0 mEq/L [3]. While hospital laboratories report "normal" serum potassium range as 3.5-5.0 mEq/L, microelectrode measurements demonstrate that cardiac cells begin depolarizing when serum potassium decreases below 4.0 mEq/L, and also cardiac cell depolarization occurs when serum potassium increases above 5.0 mEq/L [5]. Thus, hypokalemia and hyperkalemia cause electrophysiological changes in the myocardial cell that may increase atrial and ventricular spontaneous activity.
Attenuation by hypermagnesemia of the electrophysiological effects of hyperkalemia on human and canine heart cells
Because hypermagnesemia has been shown to promote maintenance of a negative resting potential, it might oppose the depolarizing effects of hyperkalemia on cardiac cells. To test this hypothesis, electrocardiographic changes that occur during hyperkalemia were studied in 11 patients, 4 of whom also had hypermagnesemia [5]. Microelectrode studies were also carried out in atrial and ventricular cells isolated from 11 canine hearts using similar extracellular concentrations of magnesium and potassium to elucidate further the relative effects of the cations. Electrocardiographic changes in 7 patients during an episode of hyperkalemia (range 7.2-8.8 mEq/L, mean 7.9 ± 0.6 mEq/L with mean serum magnesium 1.8 ± 0.2 mEq/L) was associated with a significant reduction in P wave amplitude and marked prolongation of the QRS complex. However, normal P waves and normal QRS durations were found in 4 patients with both hyperkalemia (range 6.9-7.6, mean 7.3 ± 0.2 mEq/L) and hypermagnesemia (range 3.3-6.8, mean 4.8 ± 0.2 mEq/L). Similarly, in isolated atrial and ventricular cells, hyperkalemia with normal magnesium induced depolarization; hyperkalemia induced less depolarization in the setting of elevated levels of magnesium. These combined observations support the concept that elevated extracellular magnesium attenuates the electrophysiological effects of hyperkalemia on the heart.
Torsades de pointes: indication for magnesium therapy
Torsades de pointes (turning of the points) is an uncommon and distinctive form of polymorphic ventricular tachycardia that occurs in the setting of an abnormally long QT interval [6]. While the most common cause is treatment with QT-prolonging medications, torsades de pointes can also occur in the congenital long QT syndromes and in the setting of acquired heart block or severe electrolyte disturbances, notably hypokalemia [7]. Of note, the first published episode of torsades de pointes was probably in 1935, in association with hypomagnesemia [8]. Intravenous MgSO4 has proven to be extremely effective in terminating this serious rhythm disturbance and is now regarded as the treatment of choice.
With an increasing awareness of the phenomenon of drug-induced torsades de pointes came recognition of some factors that appear to confer increased risk for the arrhythmia [6]. It is recognized that QT intervals are longer in women than in men although the mechanism is not well understood. Of note, in some series of torsades de pointes, there is a two-threefold higher incidence of the arrhythmia among women. This includes not only drug-induced cases but also extends to the congenital long QT syndrome, in which syncope is more common among women who carry long QT mutations than among men who carry mutations. In most series, hypokalemia and hypomagnesemia are common among patients who develop drug-induced torsades de pointes. It is not well-established whether the type of underlying heart disease affects the incidence of torsades de pointes, although there is some suspicion that left ventricular hypertrophy or congestive heart failure may increase the risk. The most important principle in the treatment of torsades de pointes is to recognize the arrhythmia. All QT-prolonging drugs should be withdrawn, and hypokalemia should be corrected. In vitro studies suggest that potassium will not only shorten the QT interval but may also decrease the potency of QT-prolonging drugs. Thus, serum potassium should be kept in the high normal range. Intravenous administration of 1 to 2 grams of MgSO4 over 1 to 2 minutes appears to prevent recurrence of torsades de pointes in most patients. It is interesting that while magnesium prevents episodes of torsades de pointes (limits triggered activity), it does not shorten the QT interval.
The combination of quinidine therapy, bradycardia and hypokalemia is known to predispose to torsades de pointes. Clinical reports have shown suppression of quinidine-induced torsades de pointes with intravenous administration of MgSO4. To further understand these observations, isolated Purkinje fiber preparations were studied using standard microelectrode techniques [9]. Preparations were exposed to low concentrations of quinidine and superperfused with Tyrode's solution containing normal potassium of 4.0 mEq/L and MgSO4 1.0 mEq/L with a pH of 7.4. Triggered activity appeared within 10 minutes after potassium was decreased to the 2.5-3.2 mEq/L range and was abolished when normal potassium concentration was restored. When magnesium was increased to 2.0 mEq/L, it abolished runs of spontaneous activity. In preparations in which lower concentrations of potassium (2.7-3.2 mEq/L) were used to elicit spontaneous beating, higher concentrations of MgSO4 in the 3.0-6.0 mEq/L range were needed to attenuate this spontaneous activity. High levels of magnesium greater than 6.0 mEq/L completely suppressed triggered activity in all preparations. These data show a similarity between the conditions that predispose to torsades de pointes in the clinic and the conditions under which quinidine may induce spontaneous beating in isolated Purkinje fibers. These observations of the effect of magnesium to suppress triggered activities are consistent with the thought that extracellular magnesium ions produce a membrane-stabilizing effect. The immediate resolution of torsades de pointes when intravenous MgSO4 is given also is consistent with an extracellular magnesium-stabilizing mechanism.
In summary, MgSO4 1-2 grams intravenously given over 1-2 minutes is recommended in suspected cases of torsades de pointes. The rapid infusion of magnesium for the treatment of torsades de pointes is not intended to correct a deficient state but rather is considered a pharmacologic intervention and an antiarrhythmic effect, which occurs without regard to the initial serum magnesium level [6]. Intravenous MgSO4 also should be administered for polymorphic ventricular tachycardia with suspected hypomagnesemia [10]. However, it is not recommended as a routine strategy for cardiac arrest.
Effect of High Doses of Magnesium on Making Ibutilide a Safer and More Effective Agent
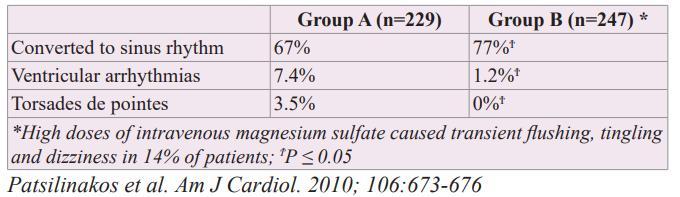
The most serious complication of ibutilide administration is torsades de pointes due to QT prolongation, which would preclude its use in a non-monitored environment. Animal data strongly suggest that the risk of torsades de pointes during administration of an intravenous QT-prolonging drug may be increased by increasing the rate of infusion [11,12]. Thus, clinicians using ibutilide should not administer the drug any more rapidly than the 1 mg intravenously over 10 minutes recommended by the manufacturer. Torsades de pointes occurs in approximately 4% of patients receiving ibutilide. MgSO4 (1-4 grams IV) has been successfully used for the treatment of torsades de pointes. Prophylactic administration of intravenous MgSO4 appears to prevent the increases in QT and QTc interval after the last infusion of ibutilide and to protect against the development of torsades de pointes. Ibutilide is a class III antiarrhythmic agent indicated for cardioversion of atrial fibrillation and atrial flutter to sinus rhythm. While magnesium has been successfully used for the treatment of torsades de pointes, its use as a prophylactic agent for this arrhythmia has not been fully established. Investigators have examined whether high doses of magnesium would increase the safety and efficacy of ibutilide administration in patients with recent onset (previous 48 hours) atrial fibrillation or atrial flutter who were candidates for conversion to sinus rhythm [13]. Group A consisted of 229 patients who received ibutilide to convert atrial fibrillation or atrial flutter to sinus rhythm. Group B consisted of 247 patients who received an intravenous infusion of 5 grams of MgSO4 (40.6 mEq of elemental magnesium) for 1 hour followed by the administration of ibutilide. Then, another 5 grams of MgSO4 were infused for 2 additional hours. No differences were seen in age, gender or other characteristics between the patients who received only ibutilide than those who also received intravenous MgSO4. High doses of intravenous MgSO4 (10 grams over 3 hours) caused transient flushing, tingling and dizziness in 14% of patients but overall was well tolerated. As shown in Table 1, the administration of high doses of MgSO4 significantly increased the conversion efficacy of ibutilide and appears to make ibutilide a much safer agent with significantly less ventricular arrhythmias and no torsades de pointes. Thus, magnesium supplementation, although not yet established from double-blind controlled trials, might convert ibutilide to a much safer agent and could be used as a routine co-therapy in patients receiving ibutilide for cardioversion of recent onset atrial fibrillation or atrial flutter. This study likely reflects the stabilizing effect of extracellular hypermagnesemia on the conduction system of the heart.
Table 1: Effect of High Doses of Magnesium Making Ibutilide a Safer and More Effective Agent for Cardioversion of Recent Onset Atrial Fibrillation or Atrial Flutter to Sinus Rhythm.
Differential Effects of Intravenous Magnesium onAtrioventricular
Node Conduction in Supraventricular Tachy cardia
Further insight into the effects of extracellular magnesium on the electrical system of the heart can be gained by a study of 23 patients exploring the electrophysiological effects of intravenous MgSO4 on supraventricular tachycardia with particular reference to atrioventricular (AV) node pathways [14]. Measurements were made before and after administering 2.47 grams of MgSO4 (20 mEq of elemental magnesium) intravenously over 1 minute. Serum magnesium rose from 1.76 mEq/L to 3.58 mEq/L. At this level, extracellular magnesium prolonged the AH (Atrio-His) interval during tachycardia and thereby increased tachycardia cycle length to a greater extent in patients with evidence of dual AV node physiology, and the dominant effect of magnesium was on the antegrade slow pathway conduction. It is not surprising, given the different conduction refractory characteristics of fast and slow AV node pathways, that they may be affected differently by pharmacologic interventions like increasing extracellular magnesium. It has been suggested that the different conduction refractory characteristics of fast and slow AV nodal pathways may be a result of differing populations of membrane channels at each site. L-type calcium channels are known to be essential for AV conduction, and increases in magnesium concentration have been reported to decrease peak L-type calcium current. This is likely to be a result of decreased channel availability via gaiting kinetics although direct block of open channels by magnesium may play an additional role. This study also demonstrates extracellular magnesium membrane stabilizing effects at safe concentrations which are the same concentrations used in microelectrode studies using isolated Purkinje fibers.
Information about serum (extracellular) magnesium and implications of acute changes independent of total body magnesium
In healthy subjects with stable renal function and eating their usual diet, serum magnesium remains stable with minor biological variations. However, serum magnesium has been found to increase with maximal treadmill exercise testing [15]. In 15 sedentary, healthy men (mean age 29 years), serum magnesium levels increased significantly (1.7 mEq/L to 1.8 mEq/L; p < 0.01) at peak exercise whether the patients were on selective beta-blocker atenolol, nonselective beta-blocker propranolol or placebo. There was no difference among groups in baseline recovery for magnesium (mean 28 minutes, range 24-30 minutes). In conclusion, serum magnesium levels increase significantly with maximal exercise and are unaffected by atenolol or propranolol beta-blockade. In this study, the increase in serum magnesium with exercise may reflect a "protective role” with regard to arrhythmias that may be provoked by exercise.
Hypomagnesemia
Acute stressor states are linked to neurohumoral activation that includes increased sympathetic nervous system activity. Elevations in circulating epinephrine and norepinephrine unmask an interdependency that exists between potassium and magnesium based on their regulation of a large number of magnesium- dependent Na+/K+-ATPase pumps present in skeletal muscle. The hyperadrenergic state accounts for a sudden translocation of cations into skeletal muscle with rapid appearance of hypokalemia and hypomagnesemia [16]. The resultant hypokalemia and hypomagnesemia cause a delay in myocardial repolarization and electrocardiographic QTc prolongation increasing the propensity for supraventricular and ventricular arrhythmias.
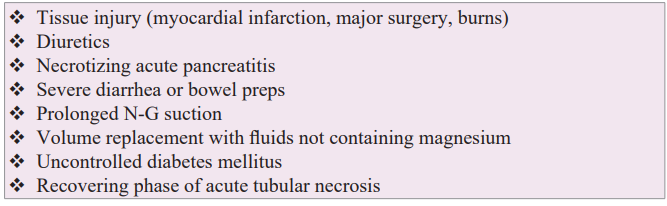
Magnesium is the second most prevalent intracellular cation in the human body. The normal adult body contains about 25 grams of magnesium divided almost equally between the skeletal and soft tissue cells [1,2]. Less than 1% of the body's magnesium is extracellular and found in the blood. Magnesium concentration in plasma is 1.5-2.2 mEq/L with about two-thirds as free cation and one-third bound to plasma proteins. About one-third of intracellular skeletal magnesium is on the surface of the bone and thought to serve as a reservoir to maintain the extracellular magnesium in the blood. When an acute decrease in serum magnesium occurs (Table 2), mobilization of magnesium cation from intracellular pool in bone to extracellular space is fairly rapid in children but not in adults and is slower than mobilization of calcium. When acute hypomagnesemia occurs, adult patients often have no symptoms, and there are no ECG abnormalities [17]. A sudden change in cardiac rhythm may be the only indicator of acute hypomagnesemia. For example, a patient undergoes an extensive operation, and 48 hours later cardiology consult is requested for onset of frequent premature ventricular contractions. Hypomagnesemia is found, and intravenous MgSO4 stabilizes the rhythm.
Magnesium's physiological role is primarily related to enzyme activity with over 300 enzyme systems dependent on its presence as a coenzyme [1]. However, extracellular magnesium also has an important role responding to and stabilizing tissue injury (plugging micropores and binding to injured membranes). The larger the tissue injury, the greater acute depletion of extracellular magnesium. As listed in Table 2, examples of tissue injury include acute myocardial infarction, extensive surgery and burns. Eighty percent of serum magnesium is filtered at the glomerulus, 15- 20% is reabsorbed in the proximal tubule, 65-70% is reabsorbed in the thick ascending limb of the loop of Henle, and 5-10% is reabsorbed in the distal tubule. When serum blood sugar is greater than 250 mg/dL, glycosuria occurs, and glycosuria inhibits the normal reabsorption of magnesium from the tubule resulting in renal loss of magnesium. Another example of renal loss of magnesium may occur during the recovering phase of acute tubular necrosis where tubular reabsorption of the filtered magnesium is ineffective. Hypomagnesemia due to lactation causing ventricular tachyarrhythmias has also been reported [18].
Table 2: Causes of Acute Extracellular Hypomagnesemia.
The FDA has warned that hypomagnesemia has occurred rarely with prolonged use of a proton pump inhibitor (PPI) and is usually accompanied by hypokalemia and hypocalcemia [19-24]. Patients also taking other medicines that cause hypomagnesemia, such as loop diuretics, may be at increased risk. The exact mechanism causing hypomagnesemia from prolonged use of a PPI is unknown, but they may interfere with magnesium absorption. When the PPI is stopped, serum magnesium returns to normal in less than two weeks. If cardiac arrhythmias are a concern, more rapid replacement is recommended [23,24].
A study that emphasized the clinical consequences of hypomagnesemia and hypermagnesemia examined the prevalence of serum magnesium alterations and outcomes in hospitalized patients at Mayo Clinic from January 1, 2009 through December 31, 2013 (288,120 patients were screened) [25]. Excluding patients less than 18 years of age, those without magnesium measurement and readmission episodes, 65,974 were studied. Magnesium levels of 1.7 mEq/L or higher were found in 20,777 patients (31.5%), and levels less than 1.4 mEq/L were found in 13,320 (20.2%). A magnesium level less than 1.4 mEq/L was independently associated with an increased risk of hospital mortality, risk of longer hospital stay and being discharged to a care facility. An elevated magnesium level of 1.9 mEq/L or higher (n = 7908) was associated with worse hospital mortality in a dose-response manner. In patients with cardiovascular disease, magnesium levels of 1.23-1.4 mEq/L and 1.9 mEq/L or higher both independently predicted poor outcomes including hospital mortality. The lowest hospital mortality occurred in patients with magnesium levels between 1.4 and 1.55 mEq/L. Thus, dysmagnesemia in hospital patients is common. Magnesium supplementation for patients without hypomagnesemia should be avoided in the absence of randomized controlled trials documenting a benefit.
In these clinical scenarios, checking serum magnesium levels and monitoring for cardiac rhythm changes is recommended.
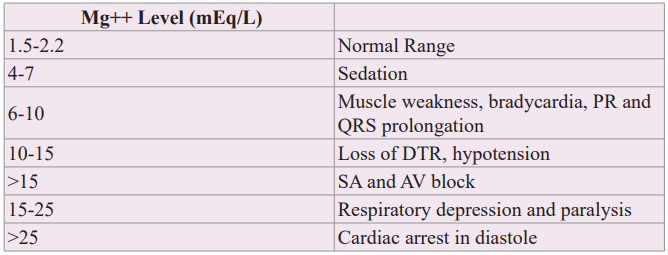
Hypermagnesemia
Table 3 describes the clinical, ECG and cardiac rhythm responses to increasing levels of serum magnesium. A serum magnesium level of 4-7 mEq/L provides sedation in the treatment of preeclampsia. As shown in microelectrode studies of canine sinus node and Purkinje cell preparations, hypermagnesemia of 6-10 mEq/L causes bradycardia and PR and QRS prolongation [3,5]. Loss of deep tendon reflexes is an important clinical finding to suspect serum magnesium levels in the 10-15 mEq/L range. When the serum magnesium level is greater than 15 mEq/L, heart block occurs with cardiac arrest in diastole at greater than 25 mEq/L. Common causes of hypermagnesemia are magnesium-containing antacids or laxatives taken by patients with acute or chronic renal failure [26]. With readily available serum magnesium measurements, early detection of hypermagnesemia is ideal to avoid serious outcomes.
Digitalis Toxicity
Indications for digitalis use have decreased over the years. Hypomagnesemia can potentiate certain digitalis toxic arrhythmias. Hypomagnesemia is usually associated with hypokalemia [27]. Diuretics by virtue of causing hypokalemia and hypomagnesemia may predispose the patient to digitalis toxicity. Digitalis toxicity has also been found in patients with hypomagnesemia and normokalemia [28-30]. To determine whether hypomagnesemia is facilitating the development of digitalis toxicity in the absence of hypokalemia, studies have been performed in adult Mongrel dogs [31]. Hypomagnesemia was achieved by Kiil kidney dialysis. Mean serum magnesium was reduced 44% from 1.4 to 0.76 mEq/L. This was accompanied by a 26% reduction in the amount of acetyl- strophanthidin needed to produce toxic arrhythmias. Restitution of sinus rhythm was observed after intravenous infusion of MgSO4. These studies demonstrate that hypomagnesemia, even with normokalemia, facilitates digitalis toxicity. Since diuretic drugs may produce hypomagnesemia as well as hypokalemia and both may predispose to digitalis toxicity, it is suggested that serum magnesium as well as potassium levels be measured periodically in patients on diuretic therapy.
Atrial fibrillation
There have been many studies examining the relationship between hypomagnesemia and atrial fibrillation. Low serum magnesium and the development of atrial fibrillation in the community was examined in the Framingham Heart Study with an over 20- year followup [32]. Low serum magnesium was found to be moderately associated with the development of atrial fibrillation in individuals without cardiovascular diseases. The association between serum magnesium and atrial fibrillation was not linear but suggested a threshold of serum magnesium equal to or less than 1.5 mEq/L. Magnesium and potassium deficiency frequently coexist. Because of the association between magnesium and potassium, the Framingham investigators performed secondary analysis adjusting for serum potassium, and the magnesium- atrial fibrillation relationship remained unchanged. Furthermore, baseline serum potassium was unrelated to incidence of atrial fibrillation in the Framingham data. Thus, it seemed unlikely that potassium concentrations explained the serum magnesium- atrial fibrillation association. These results have important public health implications because the prevalence of atrial fibrillation is increasing, and magnesium deficiency is common and potentially modifiable.
Clinical studies have shown that intravenous MgSO4 is an effective and safe strategy for the acute management of atrial fibrillation with a rapid ventricular response [33,34]. Further, keeping serum magnesium greater than 2.0 mEq/L has been shown to improve rate control in the treatment of chronic atrial fibrillation and facilitate maintenance of sinus rhythm [35]. It has also been observed that hypomagnesemia increases the dose of digoxin required for rate control of atrial fibrillation and lowers the threshold for digitalis-induced arrhythmias [28].
Hypomagnesemia is common following cardiac surgery because initiation of extracorporeal circulation during surgery may dilute the circulating blood volume. Hypomagnesemia may also occur after cardiac surgery due to tissue injury with delay in magnesium response from intracellular stores and because use of diuretics after surgery will promote urinary excretion of magnesium. Low serum magnesium is associated with the development of atrial fibrillation after coronary artery bypass grafting (CABG). Some, but not all, studies suggest that magnesium supplementation reduces the incidence of postoperative atrial fibrillation. In a meta-analysis 17 randomized control trials (n = 2069 patients) testing whether magnesium supplementation reduced the incidence of arrhythmia after cardiac surgery, pooled mean serum magnesium at 24 hours after surgery in the treatment group was significantly higher at 1.18 mEq/L than in the control group at 0.9 mEq/L [36]. Administration of prophylactic magnesium reduced the risk of atrial fibrillation by 29% and ventricular arrhythmias by 48%. Magnesium supplementation had no noticeable benefit with respect to length of hospitalization, instance of myocardial infarction or mortality. On pharmacologic analysis of these studies, supplementation per se was not the defining factor, but achieving a serum magnesium level of greater than 2.0 mEq/L appeared effective. This again suggests a threshold effect and guide for magnesium replacement therapy.
Inappropriate sinus tachycardia (resting heart rate greater than 100 beats per minute)
Inappropriate sinus tachycardia (IST) usually affects younger female individuals between the ages of 15 and 45 years with four times higher prevalence than men and is associated with distressing symptoms which may affect quality of life, including palpitations, anxiety, dizziness, presyncope and syncope [37]. IST is a diagnosis of exclusion in that these patients are euthyroid, afebrile, not anemic and have no evidence for heart failure. The pathogenesis of IST is not well understood and is considered multifactorial, with autonomic dysfunction being the central abnormality. A recent prospective study reported a prevalence of 20% after COVID-19 infection. Avoiding stimulants, including caffeine and nicotine, is now recommended. Increasing vagal activity via yoga and regular aerobic exercise (walking and bicycling) lowers resting heart rate. Beta-blockers and calcium channel blockers have limited efficacy because of hypotension. Ivabradine, an inhibitor of the funny current (If) has shown promise in reduction in heart rate and improvement in symptoms. Of note, ivabradine is contraindicated in pregnant patients and breastfeeding mothers, and access to this drug has been limited by supply chain issues and high cost in the United States. Recent advances in catheter and surgical sinus node- sparing ablation techniques have led to improvement in outcomes. Checking for hypomagnesemia as a contributing mechanism to IST and/or treating IST with chronic magnesium supplements warrants study in double-blind controlled trials.
Low serum magnesium and cardiovascular mortality in chronic heart failure patients
Long-term effects of low serum magnesium in chronic systolic (HFrEF) and diastolic (HFpEF) heart failure patients with normal sinus rhythm were examined in a propensity-matched study [38]. Propensity scores for having low serum magnesium levels were calculated for each patient using a non-parsimonious multivariant logistical regression model and were used to match 560 low magnesium (less than 2.0 mEq/L) patients with 560 normal magnesium (greater than 2.0 mEq/L) patients during a mean followup of 36 months. On analysis, ambulatory, chronic heart failure patients with serum magnesium levels less than 2.0 mEq/L were found to have increased cardiovascular mortality but had no association with cardiovascular hospitalizations. Based on these results, when low serum magnesium is detected in heart failure patients, they should be treated with magnesium supplements and/ or an aldosterone antagonist with the goal being to keep serum magnesium at 2.0 mEq/L or greater.
Magnesium in acute ST elevation myocardial infarction (STEMI)
Patients with acute ST elevation myocardial infarction or STEMI may have a total body deficit of magnesium because of a low dietary intake, advanced age and prior diuretic use [1]. They may also acquire a functional extracellular magnesium deficit due to increased sympathetic nervous system activity with the onset of a myocardial infarction and/or acute hypomagnesemia due to tissue damage [2,16]. The ISIS-4 investigators enrolled 58,050 patients, 29,011 to magnesium and 29,039 to control [39]. The control group mortality was 7.2% in ISIS-4 compared with 7.6% in the magnesium group. The MAGIC trial investigated the benefit of early administration of intravenous magnesium to high-risk patients with acute STEMI [40]. At 30 days, the mortality rate was 15.3% in the magnesium group and 15.2% in the placebo group (p = 0.96). Between 1980 and 2002, 68,684 patients were studied in a series of 14 randomized trials. Based on the totality of available evidence, there is no indication for the routine administration of intravenous MgSO4 to patients with acute STEMI at any level of risk. However, because of the risk of cardiac arrhythmias when electrolyte deficits are present in the early phase of myocardial infarction, all patients with acute STEMI should have a serum magnesium and potassium measurement on admission [41,42]. It is recommended to replace magnesium deficits to maintain a serum magnesium level of 2.0 mEq/L or more. During the course of treatment of STEMI, the serum magnesium level should be rechecked and replaced.
Hemodynamic and antiarrhythmic effects of intravenous magnesium sulfate
Magnesium sulfate is the most widely available intravenous preparation and almost exclusively used in many of the studies cited in this review. Intravenous magnesium has an excellent safety profile. Serum levels may be transiently elevated for a few hours after administration of an intravenous dose. The rate of intravenous magnesium administration depends on the particular indication. However, caution is advised in patients with renal insufficiency. To avoid serious adverse effects of intravenous MgS04, bolus doses should not exceed 6 grams (48.7 mEq of elemental magnesium) over a 15-20-minute period, and continuous infusion rates should not exceed 3 grams (24.4 mEq of elemental magnesium) per hour. Intravenous MgS04 is an effective way to rapidly increase extracellular magnesium. In 23 patients, 2.47 grams of MgSO4 (20 mEq of elemental magnesium) administered intravenously over 1 minute raised serum magnesium from 1.76 mEq/L to 3.58 mEq/L with subjects experiencing transient flushing [14]. After termination, sinus rate and blood pressures (128/81 mmHg ± 22/13 to 121/78 ± 22/11; p = 0.70) were unaffected. The effect of MgSO4 infusion on coronary and systemic hemodynamics on the normal human heart has also been studied [43]. Four grams of MgSO4 (32.5 mEq of elemental magnesium) were given intravenously over 10 minutes in 9 patients with normal coronary arteries. Mild cutaneous flushing was observed; no hypotension, bradycardia or AV ventricular or intraventricular conduction disturbances were observed. The main finding of the study was the vasodilator effect of MgSO4 on the coronary arterial bed. There was a similar proportional increase in systemic (23%) and coronary blood flow (22%) after MgSO4. The mechanism of this MgSO4-induced systemic and coronary dilatation is probably related to the calcium antagonistic effect of extracellular magnesium ions at the level of the vascular smooth muscle. When giving intravenous MgSO4 to treat a ventricular arrhythmia, improved coronary blood flow may be of benefit if myocardial ischemia was a trigger for ventricular ectopy.
Table 4: Possible indications for intravenous magnesium as an anti-arrhythmic agent.
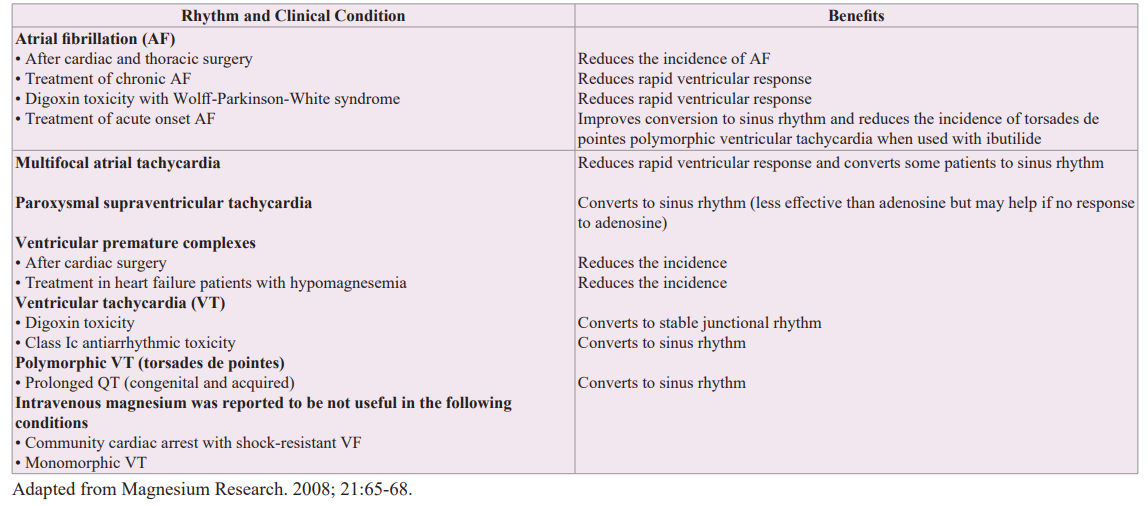
Intravenous magnesium has been shown to have a number of electrophysiological actions on the conduction system of the heart, and the mechanism of its extracellular actions may include calcium antagonism at the L- and T-type calcium channels [44]. In supraphysiologic doses, extracellular magnesium decreases the outward potassium current density resulting in membrane stabilization. Magnesium also acts as an indirect antagonist of digoxin at the sarcolemma Na+-K+-ATPase pump. Table 4 lists possible indications of magnesium as an antiarrhythmic agent [44-48]. The strongest indication is polymorphic VT (torsades de pointes) [6]. Of note, magnesium should not be given before cardiac surgery because its extracellular stabilizing effects have been found to make restarting the heart after cardioplegia more difficult. Further, intravenous magnesium has been reported not to be useful in cardiac arrest with shock-resistant ventricular fibrillation or in monomorphic ventricular tachycardia [49].
Apart from being effective in controlling many cardiac arrhythmias, Intravenous magnesium has little detrimental hemodynamic effects on the cardiovascular system although minor symptoms of flushing, tingling and dizziness are common. Evidence that supports use of intravenous magnesium in many different types of cardiac arrhythmias is however based on case studies, animal studies and randomized controlled trials that evaluated physiological endpoints only [44]. None of the published randomized controlled trials on intravenous magnesium has demonstrated a significant reduction in mortality.
Summary and Conclusions
Changes in extracellular magnesium is an important mechanism by which this cation independent of total body magnesium exerts its effect on the electrical system of the heart. Hypomagnesemia depolarizes heart cells resulting in both supraventricular and ventricular tachyarrhythmias. An increase in sinus rate may be the most common rhythm seen with hypomagnesemia. In contrast, hypermagnesemia hyperpolarizes heart cells suppressing atrial and ventricular arrhythmias. Intravenous MgSO4 is safe in recommended doses and is the initial recommended therapy for torsades de pointes. Monitoring for cardiac rhythm changes and measuring serum magnesium concentrations is recommended for patients in clinical situations where extracellular magnesium may increase or decrease rapidly. Supplemental magnesium levels greater than 2.0 mEq/L may help in rate control in patients with atrial fibrillation and a rapid ventricular response and may decrease the incidence of atrial fibrillation after CABG. Measuring serum magnesium and replacing to greater than 2.0 mEq/L is also recommended in patients on digitalis, in patients post-acute myocardial infarction and in patients on diuretic therapy for both HFrEF and HFpEF. Measuring an eGFR to detect renal insufficiency is also important in patient management to avoid hypermagnesemia during magnesium replacement therapy.
References
- Mosley. Deficiency #4: Magnesium. Cortlandt Forum. 2008; 25-26.
- Rude RK. Magnesium deficiency: a cause of heterogeneous disease in humans. J Bone Minor Res. 1998; 13: 749-758.
- Woods WT, Katholi RE, Urthaler Fm, et al. Electrophysiological effects of magnesium on cells in the canine sinus node and false tendon. Circ Res. 1979; 44: 182-188.
- Katholi RE, Woods WT, Kawamura K, et al. Dual dependence on both Ca2+ and Mg2+ for electrical stability in cells of canine false tendon. J Mol Cell Cardiol. 1979; 11: 435-445.
- Kraft LF, Katholi RE, Woods WT, et al. Attenuation by magnesium of the electrophysiologic effects of hyperkalemia on human and canine heart cells. Am J Cardiol. 1980; 45: 1189-1195.
- Roden DM. A practical approach to torsades de pointes. Clin Cardiol. 1997: 20: 285-290.
- Vincent GM, Timothy KW, Leppert M, et al. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med. 1992; 327: 846-852.
- Zwillinger L. Über die. Magnesiumwirkung auf das Herz. Klinische Worhenschr. 1935; 40: 1429-1433.
- Davidenko JM, Cohen L, Goodrow R, et al. Quinidine-induced action potential prolongation, early afterdepolarizations, and triggered activity in canine Purkinje fibers. Effects of stimulation rate, potassium, and magnesium. Circulation. 1989; 79: 674-686.
- Roden DM. Magnesium treatment of ventricular arrhythmias. Am J Cardiol. 1989; 63: 43-46.
- Carlsson L, Abrahamson C, Andersson B, et al. Proarrhythmic effects of the class III agent almokalant: importance of infusion rate, QT dispersion, and early afterdepolarisations. Cardiovasc Res. 1993; 27: 2186-2193.
- Yang T, Roden DM. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996; 93: 407-411.
- Patsilinakos S, Christou A, Kafkas N, et al. Effect of high doses of magnesium on converting ibutilide to a safe and more effective agent. Am J Cardiol. 2010; 106: 673-676.
- Stiles MK, Sandra P, Patrick Disney, et al. Differential effects of intravenous magnesium on atrioventricular node conduction in supraventricular tachycardia. Am J Cardiol. 2007; 100: 1249-1253.
- Fletcher GF, Sweeney ME, Fletcher BJ. Blood magnesium and potassium alterations with maximal treadmill testing: effects of B-adrenergic blockade. Am Heart J. 1991; 121: 105-110.
- Nayyon M, Yusef J, Khan MU, et al. K+ and Mg2+ dyshomeostasis in acute hyperadrenergic stressor states. Am J Med Sci. 2017; 353: 422-424.
- Surawicz B. Is hypomagnesemia or magnesium deficiency arrhythmogenic?. J Am Coll Cardiol. 1989; 14: 1093-1096.
- Mela T, Galvin JM, McGovern BA. Magnesium deficiency during lactation as a precipitant of ventricular tachyarrhythmia. Pacing Clin Electrophysiol. 2002; 25: 231-233.
- Regolisti G, CabassiA, Parenti E, et al. Severe hypomagnesemia during long-term treatment with a proton pump inhibitor. Am J Kidney Dis. 2010; 56: 168-174.
- Hoorn EJ, van der Hoek J, de Man RA, et al. A case series of proton pump inhibitor-induced hypomagnesemia. Am J Kidney Dis. 2010; 56: 112-116.
- In Brief: PPI’s and hypomagnesemia. Med Lett Drugs Ther. 2011; 53:25.
- Kieboom BCT, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population-based cohort study. Am J Kidney Dis. 2015; 66: 775-782.
- In brief: PPIs and Torsades de Pointes. Med Lett Drugs Ther. 2016; 58: 153.
- Safety of long-term PPI use. Med Lett Ther. 2017; 59: 131-133.
- Cheungpasitporn W, Thongprayoon C, Qian Q. Dysmagnesemia in hospitalized patients: prevalence and prognostic importance. Mayo Clin Proc. 2015; 90: 1001-1010.
- Bokhari SR, Siriki R, Federico JT, et al. Fatal hypermagnesemia due to laxative use. Am J Med Sci. 2018; 355: 390-395.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007; 18: 2649-2652.
- Seller RH, Cangiano J, Kim KE, et al. Digitalis toxicity and hypomagnesemia. Am Heart J. 1970; 79: 57-68.
- Seller RH. The role of magnesium in digitalis toxicity. Am Heart J. 1971; 82: 551-556.
- Neff MS, Mendelssohn S, Kim KE, et al. Magnesium sulfate in digitalis toxicity. Am J Cardiol. 1972; 29: 377-382.
- Beller GA, Hood WB, Smith TW, et al. Correlation of serum magnesium levels and cardiac digitalis intoxication. Am J Cardiol. 1974; 33: 225-229.
- Khan AM, Lubitz SA, Sullivan LM, et al. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2013; 127: 33-38.
- Brodsky MA, Orlov MV, Capparelli EV, et al. Magnesium therapy in new-onset atrial fibrillation. Am J Cardiol. 1994; 73: 1227-1229.
- Onalan O, Crystal E, Daoulah A, et al. Meta-analysis of magnesium therapy for the acute management of rapid atrial fibrillation. Am J Cardiol. 2007; 99: 1726-1732.
- Frick M, Östergren J, Rosenqvist M. Effect of intravenous magnesium on heart rate and heart rate variability in patients with chronic atrial fibrillation. Am J Cardiol. 1999; 84: 104- 108.
- Shiga T, Wajima Z, Inoue T, et al. Magnesium prophylaxis for arrhythmias after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Med. 2004; 117: 325-333.
- Ahmed A, Venkata N, Pothineni K, et al. Inappropriate sinus tachycardia: etiology, pathophysiology, and management: JACC Review Topic of the Week. J Am Coll Cardiol. 2022; 79: 2450-2462.
- Adamopoulos G, Pitt B, Sui X, et al. Low serum magnesium and cardiovascular mortality in chronic heart failure: a propensity-matched study. Int J Cardiol. 2009; 136: 270-277.
- ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group: ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet. 1995; 345: 669-685.
- Magnesium in Coronaries (MAGIC) Trial Investigators. Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) Trial: a randomised controlled trial. Lancet. 2002; 360: 1189-1196.
- Harris AS, Estandia A, Smith HT, et al. Magnesium sulfate and chloride in suppression of ectopic ventricular tachycardia accompanying acute myocardial infarction. Am J Physiol. 1953; 172: 251-258.
- Peacock JM, Ohira T, Post W, et al. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010; 160: 464-470.
- Vigorito C, Giordano A, Ferraro P, et al. Hemodynamic effects of magnesium sulfate on the normal heart. Am J Cardiol. 1991; 67: 1435-1437.
- Ho KM. Intravenous magnesium for cardiac arrhythmias: jack of all trades. Magnesium Res. 2008; 21: 65-68.
- DiCarlo LA Jr, Morady F, de Buitleir M, et al. Effects of magnesium sulfate on cardiac conduction and refractoriness in humans. J Am Coll Cardiol. 1986; 7: 1356-1362.
- Wesley RC Jr, Haines DE, Lerman BB, et al. Effect of intravenous magnesium sulfate on supraventricular tachycardia. Am J Cardiol. 1989; 63: 1129-1131.
- McCord JK, Borzak S, Davis T, et al. Usefulness of intravenous magnesium for multifocal atrial tachycardia in patients with chronic obstructive pulmonary disease. Am J Cardiol. 1998; 81: 91-93.
- Sideris AM, Galiatsu E, Filippatos GS, et al. Effects of magnesium and potassium of Wolff-Parkinson-White syndrome. J Electrocardiol. 1996; 29: 11-16.
- Allen BJ, Brodsky MA, Capparelli EV, et al. Magnesium sulfate therapy for sustained monomorphic ventricular tachycardia. Am J Cardiol. 1989; 64: 1202-1204.