Comparison Between Ketamine and Dexmedetomidine in Achieving Opioid Sparing Sedoanalgesia in Patients Following Open Heart Surgery: A Randomized Clinical Trial
Author'(s): Mohamed Ahmed Hamed1*, Mostafa Mohammed Elhamamsy2, Ahmed Mohammed Aldemerdash2, Walid Saad Taha3, Ismail Sayed Aldeab4, Mahdy Ahmed Abdelhady1, Abeer Shaban Goda1, Omar Sayed Fargaly1, Rana Ahmed Abdelghaffar1, Mohamed Ahmed Shawky1, Alyaa Abdel Sattar Mohammed Hassan1, Mina Mahrous Sobhy1, Mohamed Hasan Ragab1, Mohammad Fouad Algyar5 and Yasser Salem Mostafa1
1Department of Anesthesiology, Faculty of Medicine, Fayoum University, 63514, Fayoum, Egypt.
2Department of Anesthesiology, King Saud University, Riyad, Saudi Arabia.
3Department of Anesthesiology, Faculty of Medicine, Cairo University, Cairo, Egypt.
4Department of Anesthesiology, Kobry Alkoppa Military Hospital, Cairo, Egypt.
5Department of Anesthesiology, Surgical Intensive Care and Pain Medicine, Faculty of Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt.
*Correspondence:
Mohamed Ahmed Hamed, Department of Anesthesiology, Faculty of Medicine, Fayoum University, 63511, Fayoum, Egypt, Tel: +201118560065
Received: 05 May 2024; Accepted: 27 Jun 2024; Published: 03 Jul 2024
Citation: Mohamed Ahmed Hamed, Mostafa Mohammed Elhamamsy, Ahmed Mohammed Aldemerdash, et al. Comparison Between Ketamine and Dexmedetomidine in Achieving Opioid Sparing Sedoanalgesia in Patients Following Open Heart Surgery: A Randomized Clinical Trial. Anesth Pain Res. 2024; 8(3): 1-8.
Abstract
Background: Opioid-sparing sedoanalgesia relies on non-opioid multimodal analgesic and sedative agents. It is being developed to decrease or eliminate opioid use for the management of acute postoperative pain to avoid opioid complications. This study aimed to compare ketamine and dexmedetomidine for their efficiency and safety in inducing opioid-sparing sedoanalgesia in patients following open heart surgery.
Methods: Ninety adult cardiac patients who underwent open heart surgery were enrolled. They were randomly divided into three groups. Group D received a dexmedetomidine infusion of 0.1– 0.2 μg/kg/hour, group K received a ketamine infusion of 1–2 μg/kg/min while group C received a placebo as a control group. Drugs were given after the operation in the surgical intensive care unit. Total postoperative opioid consumption was the primary outcome. Richmond agitation sedation scale, visual analog pain scale, time to first analgesic request, patient satisfaction, and incidence of any complications were recorded.
Results: In this study, we found that the median (interquartile range) postoperative opioid consumption was significantly lower in groups D and K than in group C (156 (40), 140 (83), 185 (110); p < 0.001). Richmond agitation sedation scale was statistically significant until 6 hours postoperative (p-value: < 0.05). Patient satisfaction score was higher in groups D and K than in group C (p-value: 0.005). On the other side, there is no statistically significant difference between the three study groups regarding time to first analgesic request (minutes) (p-value: 0.064) or visual analog pain scale (p-value: > 0.05). Incidence of complications was highly significant between the study groups (p-value: < 0.001).
Conclusions: To decrease postoperative opioid use with their multiple side effects and improve patient satisfaction scores in patients undergoing open heart surgery under general anesthesia, the use of either ketamine or dexmedetomidine is recommended in the postoperative period as an adjuvant modality and a good idea with observation of possibility of some preventable side effects.
Keywords
Introduction
Postoperative pain management following open heart surgery is a crucial factor for a successful outcome. Inadequate control of postoperative pain has been associated with many short- and long- term complications such as difficulty in weaning from mechanical ventilation, prolonged intensive care unit (ICU) stay, and hospital length of stay due to difficulty of ambulation and rehabilitation, with long-term cognitive impairment, and chronic pain syndromes [1-3].
In many surgical intensive care units, opioids are still the main agents used for the management of acute postoperative pain (APP), however, this trend is decreasing due to the numerous side effects of opioids [4,5]. In many evidence-based medical trials, opioid-sparing or opioid-free analgesia that relies on non-opioid multimodal analgesic agents is being developed to decrease or eliminate opioid use for the management of APP [6]. The optimal multimodal opioid-sparing analgesic technique is considered one of the most important enhanced recovery after surgery (ERAS) interventions that mitigate the undesirable effects of the surgical stress response [7]. Implementation of ERAS has been shown to reduce postoperative complications and shorten the hospital length of stay [7,8].
Ketamine is an N-methyl-D aspartate (NMDA) receptor antagonist that provides dissociative anesthesia [9]. Ketamine produces dose-related unconsciousness and analgesia with minimal effect on the central respiratory drive while stable hemodynamics are maintained [10]. Recently, ketamine has been used as an adjunct analgosedative agent for sedation and analgesia in critically ill and mechanically ventilated patients in medical and surgical ICU,and it appeared to be feasible and safe with no negative impact on outcomes, including hemodynamics [11], and ketamine use may be linked to a reduction in vasopressor requirements [12]. Also, ketamine could prove to be a beneficial adjunct in preventing chronic poststernotomy pain and depression, which occur at relatively high rates after cardiac surgery [13,14].
Regarding dexmedetomidine, it is a sedative/ hypnotic, acting within the locus coeruleus to decrease central noradrenergic activity [15]. In addition to its sedative effect, it is a mild analgesic and accentuates opioid receptor sensitivity [16].
This study aimed to compare ketamine and dexmedetomidine for their efficiency and safety in inducing opioid-sparing analgesia and sedation in patients following open heart surgery. In this study, we hypothesize that by using continuous infusion of ketamine or dexmedetomidine in addition to NSAIDs, we can reduce or eliminate opioid use for the management of APP in adult patients following open heart surgery.
Methods
This prospective randomized clinical study was registered at ClinicalTrials.gov under the NCT05474183 number (principal investigator: Mohamed Ahmed Hamed), registration date: 26/7/ 2022; (https://classic.clinicaltrials.gov/ct2/show/NCT05474183).
The study was performed from May 2022 through March 2023. After approval of our local ethical committee (D294), and based upon written informed consent, ninety cardiac patients with American Society of Anesthesiology physical status III or IV, were admitted to the coronary care unit for postoperative management following open heart surgery.
Inclusion criteria were patients aged 18 – 65 years with ejection fraction > 35% scheduled for elective isolated coronary artery bypass grafting, valve surgery or atrial septal defect, cross-clamp time ≤ 90 min, and Cardiopulmonary bypass time ≤ 120 min.
Patients with poor left ventricular function with intra-aortic balloon pump support, recent myocardial infarction (last seven days), combined procedure (i.e., coronary artery bypass grafting plus other heart/vascular procedure), emergency surgery, redo surgery, hepatic or renal failure, creatinine >1.5, history of neurological disorders or convulsions were excluded from the study.
Randomisation and Blinding
On arrival in the surgical ICU (SICU), patients were allocated randomly in a 1:1:1 ratio, using an interactive voice-response system and a computer-generated schedule sealed envelope, to receive IV infusions of either dexmedetomidine, ketamine, or control.
The trial was double-blind, with the participant, clinical care team, and assessor (research nurse or trained member of clinical staff) blinded to treatment allocation. Allocation concealment was achieved using a centralised web-based randomisation system, with treatment allocation revealed solely to an unblinded trial investigative pharmacist for drug dispensation. Trial medication for the three arms was supplied in a matching syringe pump of identical dosage strengths, and volume.
Anesthesia Technique
The main goal of the anesthesia technique was to ensure a rapid return of consciousness and protective reflexes, with minimal residual sedative effects, and importantly, it should facilitate early ambulation.
The anesthesia technique was composed of the following 10 steps:
- Premedication with oral pregabalin 75 mg the night before
- For induction and maintenance of anesthesia:
− Midazolam 0.02- 0.05 mg/kg bolus,
− Fentanyl (cumulative dose 5–15 μg/kg), followed by continuous infusion of fentanyl; 2–3 μg/kg/h as a maintenance dose,
− Rocuronium: (0.6–1.0 mg/kg – intubation dose, followed by continuous
− Infusion of Rocuronium; 0.075–0.15 mg/kg/h as a maintenance dose, and
− Sevoflurane in a dose of 0.8–1.0 (minimum alveolar concentration).
- Ventilation: Lung protective ventilation (tidal volume 6 ml/ kg predicted body weight, + PEEP:5, + FiO2 60%), and we conducted a recruitment maneuver to prevent atelectasis.
- Monitoring: Routine monitors (electrocardiogram, pulse oximetry, arterial line inserted using local anesthesia for pressure monitoring and repeated arterial blood gases, central line inserted after induction of anesthesia for monitoring central venous pressure, end-tidal carbon dioxide, core temperature through a urinary catheter, and activated clotting time (ACT) for monitoring of coagulation). Cerebral oximetry, and bispectral index (BIS) as an indicator of depth anesthesia was kept between 40 and 60.
- Cardiopulmonary Bypass (CPB): Goal-directed perfusion maintaining mean arterial pressure ≥ 60 mmHg, using Phenylephrine and Norepinephrine infusion. Additional propofol infusion (25 - 50µg/kg/min) was administered during CPB to maintain BIS between 40 and 60. Smooth conduct of CPB, with Mild hypothermia (28°C-32°C). Rewarming at the end of the procedure to achieve postoperative temperature>36 °C.
- Perioperative glycemic control: Insulin infusion, and the perioperative goal glucose ≤ 150 – 180 mEq/liter.
- Perioperative hemoglobin concentration: Goal hemoglobin transfusion trigger: 7.5 g/dl regardless of patient age and
- Protamine: Post-CPB protamine (heparin reversal) given up to the full dose (5 mg/kg after test dose) to return to baseline
- Multimodal analgesia: In addition to continuous infusion of fentanyl, at the end of surgery, paracetamol: 1 gm IV infusion over 15 min was administered with closure of the sternum, and surgical incision field block using 30 ml of Bupivacaine 0.5% just before dressing.The patient then was transferred intubated to the SICU.
- In SICU: At this step, and for opioid-sparing analgesia and sedation, using the sealed envelope technique, patients were randomly divided into two groups:
Group (K): Received ketamine infusion 1–2 μg/kg/min (0.12 mg/kg/h) titrated to the desired level of sedation.
Group (D): Received Dexmedetomidine infusion 0.1– 0.2 μg/kg/hour titrated to desired level of sedation.
Group (C): Received placebo (normal saline) infusion 0.1– 0.2 μg/kg/hour titrated to desired level of sedation.
All hemodynamic monitors used intraoperatively are continued in the SICU, and in addition, the following parameters are used to monitor the level of analgesia and sedation:During mechanical ventilation: Richmond Agitation-Sedation Scale (RASS) [17].After extubation: When the patient can explain his feelings, monitoring of the level of analgesia was done using the visual analog scale (VAS) on a pain scale of 0-10.
The additional dose of analgesia was determined by the anesthesiologist to achieve (score 0) (Alert and calm) sedation level of RASS, and/or no signs of uncontrolled pain with an acceptable pain score after extubation (VAS < 5).
In addition to the continuous infusions of study drugs, the main adjuvant therapy used for postoperative analgesia was paracetamol: 1 gm IV infusion over 15 min every 6 hours.
For control of breakthrough pain: Fentanyl 0.5 µg/kg intermittent doses.
Reversal of muscle paralysis for extubation: Neostigmine 0.5 – 0.7 mg/kg + Atropine 0.01 – 0.02 mg/kg and/or Sugammadex 2 mg/kg when the patient fulfills extubation criteria.
Extubation criteria: The patients were extubated when fulfilling the following criteria:
- Absence of active bleeding: Total chest drain output ≤ 100 mls/hour, after arrival to SICU, with ACT < 120s.
- Hemodynamic stability: Systolic blood pressure > 90mmhg, heart rate < 110 beats/min, normal sinus rhythm with no signs of low cardiac output or myocardial ischemia, with no significant inotropic or vasoactive support, with adequate urine output ≥ 0.5 ml/kg/hour.
- Normothermia: Core temperature > 36°C.
- Level of consciousness: Patient is awake, cooperative, and following simple commands (e.g., opening eyes and limb movements).
- Adequate pain relief: No signs of uncontrolled pain with acceptable VAS (On a pain scale [0-10] VAS < 5).
- Spontaneous ventilation: Respiratory rate < 25 breaths/min with Adequate Respiratory mechanics,
- Tidal volume > 5 mL/kg, Vital capacity > 6 ml/kg, O2 saturation > 95%, PaO2 > 70 with FiO2 < 50%, with PaO2/ FIO2 > 200, and PaCO2 < 50mmHg, with satisfactory post- operative chest radiograph.
If these respiratory criteria are fulfilled: We used SIMV mechanical ventilation mode with pressure support 10 cm H2O, positive ene 5 cm H2O and FiO2 ≤ 0.4, and if repeated ABG were within satisfactory range, Extubation was done within 30 minutes, but if not, we reassessed on an hourly basis. The use of standard monitoring continued until the patients were fully awake and all parameters were recorded every hour for 48 hours. After full recovery and when the patients were alert enough to express their attitude regarding the intra-procedural events, they were asked to score their level of satisfaction during the study in terms of recalling any painful or other undesirable events. A patient’s satisfaction level was assessed with a Likert five-item scoring system [18] (1= Not satisfied at all, 2= slightly satisfied, 3= somewhat satisfied, 4= very satisfied, and 5= extremely satisfied).
The primary outcome was total postoperative opioid consumption for 48 hours postoperative.
The secondary outcomes were RASS during postoperative ventilation period (recorded immediately postoperative, after 1, 2, 3, and 4 hours), RASS after extubation, VAS score, patient satisfaction and incidence of any complications (recorded immediately postoperative, after 6, 12, 24, 36, and 48 hours in the postoperative period), duration of mechanical ventilation, duration of ICU stay, total dose of fentanyl used for breakthrough pain and patient satisfaction level.
Statistical Analysis
The sample size was calculated by SPSS version 28 through power analysis by using the one-way ANOVA test to compare 3 groups using a means difference of 22 mg in opioid consumption between groups recorded in a previous study done by Lahtinen et al. [19] with pooled standard deviation 44 mg. A total of 81 patients (27 patients in each group) are required in our study to achieve a power of 90% and type 1 error of 0.05 with allocation ratio between group 1. To compensate for the possible 10% dropout, the sample size was increased to 90 patients (30 patients in each group).
Data was collected and entered into the computer using the SPSS (Statistical Package for Social Science) program for statistical analysis (Version 21). Data was entered as numerical or categorical as appropriate. Shapiro-Wilk test was used to test normality of data. When the Shapiro-Wilk test revealed no significance in the distribution of numerical variables showing normally distributed data, they were described using mean and standard deviation and parametric statistic (one -way analysis of variance (ANOVA)) was carried out and when the Shapiro-Wilk test revealed significance in the distribution of numerical variables showing non-normally distributed data, median and inter-quartile range were used for description and the non-parametric statistic (Kruskal Wallis test) was carried out. Categorical variables were described using frequency and percentage within the group. The chi-square test was used to test the association between qualitative variables. Fisher’s Exact test was carried out when indicated (expected cells less than 5). Results were considered statistically significant if the p-value was less than 0.05 level and the confidence interval was 95%.
Results
One hundred ten patients were assessed for eligibility; of those 90 patients completed the study and were randomized (30 patients for each group) between the 3 groups and their data were included in the final analysis. Twenty patients were excluded from this study on account of patients did not meet inclusion criteria (11patients) and patients’ refusals (9 patients) (Figure 1).

Figure 1: Consort flow diagram of the study population.
As shown in Table 1, no significant differences (P > 0.05) were noted between the 3 groups regarding age, weight, sex, ASA status, types of surgery, operative time, and intensive care unit stay. Postoperative ventilation time was statistically significantly lower in group D compared to K and C groups (P < 0.001) with no statistically significant differences between K and C groups (Figure 2). In Table 2, total postoperative fentanyl consumption was statistically significantly lower in group D and K compared to group C (P < 0.001). Regarding RASS score comparison during the postoperative period (Figure 2), there were statistically significantly lower scores in group D than in group K which is lower than group C during the first 6 hours postoperatively while there were no statistically significant changes after 12 hours up to 48 hours postoperatively. No statistical significance was noted between the three studied groups regarding the visual analog pain scale at all postoperative time points till 48 hours (P > 0.05). Patient satisfaction scores were statistically significant between Group D and Group K with patients extremely satisfied in Group K (P= 0.005). The incidence of complications (nausea and vomiting,heart rate changes, or blood pressure changes) was statistically significant between the studied groups (P ≤ 0.005) as shown in Table 3.
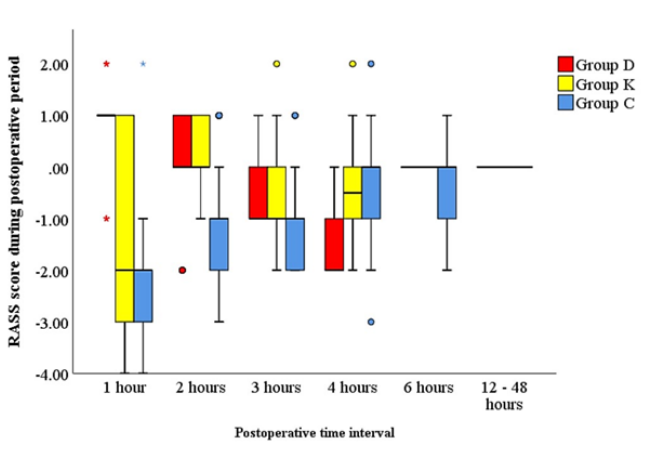
Figure 2: Clustered boxplot of RASS score during postoperative ventilation between the three study groups.
Discussion
In this randomized clinical trial that aimed to investigate the role of adjuvant analgesic agents, namely ketamine, and dexmedetomidine for opioid-sparing postoperative analgesia following adult cardiac surgery, we demonstrated that sedoanalgesia that combines sedation and analgesia through the use of dexmedetomidine or ketamine is associated with lesser opioid consumption which was our primary outcome in this study. Also, the use of these drugs was associated with lesser postoperative ventilation time, better sedation scores, and better patient satisfaction scores at the expense of some preventable side effects. No statistically significant effect appeared in intensive care unit stay, time to first analgesic request, or visual analog pain scale.
The effect of dexmedetomidine on opioid consumption can be explained by its action within the locus coeruleus to decrease central noradrenergic activity, causing a state like normal sleep within 5–10 min of infusion [20]. Also, patients remain comfortable, arousable, and can follow commands. In addition to its sedative effect, it is a mild analgesic and accentuates opioid receptor sensitivity [16]. This result is augmented by a meta-analysis performed by Schnabel reported that dexmedetomidine infusion relieved postoperative pain and reduced opioid consumption in various elective surgeries [21]. Another meta-analysis by Le Bot showed a similar reducing effect of dexmedetomidine for opioids, postoperative pain, and PONV in multiple types of elective surgery [22]. A study done by Liu et al. was the first meta-analysis to evaluate the efficiency of dexmedetomidine for opioid consumption and pain control in patients undergoing laparoscopic cholecystectomy and indicated that intravenous dexmedetomidine significantly decreased postoperative opioid consumption [23].
ketamine has a non-GABAergic mechanism of action [24]. It produces analgesia and rapid sedation by dual mechanisms mediated by inhibition of the N-methyl-D-aspartate receptor and activation of the opioid μ- and κ-receptors [25]. Additionally, ketamine provides bronchodilation, preservation of cardiac output, increase in blood pressure, and maintenance of respiratory drive and airway reflexes while actively weaning from MV [26]. These results are approved by a single-center, double-blind, randomized controlled trial (RCT) of 93 ICU post-abdominal surgery patients, adjunctive ketamine was associated with a reduced intake of morphine [27].
In concordance with our results regarding postoperative ventilation time, in a meta-analysis done by Abowali et al., it was concluded that dexmedetomidine significantly reduces the duration of mechanical ventilation and the risk of delirium across cardiac surgical ICU patients [28]. Also, Lewis et al. in their systematic review and meta-analysis reported that The use of dexmedetomidine reduced the risk of intubation and mechanical ventilation when compared with any other sedative or placebo (relative ratio, 0.54; 95% CI, 0.41-0.71; moderate certainty) [29]. These results are strengthened by Keating in his research on the use of dexmedetomidine stated that dexmedetomidine was also associated with a shorter time to extubation than midazolam and propofol, and a shorter duration of mechanical ventilation than midazolam. Patients receiving dexmedetomidine were easier to rouse, more cooperative, and better able to communicate than those receiving midazolam or propofol in their associated trials [30].
Regarding sedation scores between the study groups through the Richmond agitation sedation scale, we found a statistically significant at the first 6 postoperative hours. Dexmedetomidine sedation has been associated with easier patient rousability and preservation of cognitive performance [31]. The 2018 PADIS guidelines suggest the use of propofol or dexmedetomidine for the sedation of cardiac surgical patients, and either propofol or dexmedetomidine for medical/other surgical patients [32]. Keating in his review article on dexmedetomidine use for sedation in ICU stated that patients receiving dexmedetomidine were also easier to rouse, more cooperative, and better able to communicate than patients receiving midazolam or propofol with beneficial effects on delirium in some randomized, controlled trials and an acceptable tolerability profile concluding that it is an important option for sedation in the intensive care setting [30].
Regarding VAS scores, our results revealed a non-statistical significance between the three study groups at all time points postoperatively. These results are augmented by a meta-analysis done by Liu et al. who found that VAS scores 24 hours after the operation were reported in 452 study participants and were lower with dexmedetomidine use, but the difference was not statistically significant (p = 0.08( [23]. Also, a study done by Guillou et al. in 2003 on the effects of small-dose ketamine on morphine consumption in the surgical intensive care unit demonstrated that VAS scores at rest and mobilization for 48 hours postoperatively were similar [33].
Patients’ satisfaction scores were statistically significant in our study with better satisfaction in the ketamine group. These results are consistent with two studies of the use of ketamine in adult cardiac surgery ICU, and both showed that ketamine improved postoperative patient satisfaction, and postoperative recovery and decreased the incidence of shivering, nausea, and vomiting after CABG surgery [34,35]. As regards patient satisfaction and the opioid-sparing effect of ketamine in cardiac surgery, one study has proved that patient satisfaction was improved, and there was significant postoperative opioid-sparing with ketamine use [19].
Regarding the incidence of complications, there was a statistically significant effect between study groups in all recorded complications (p < 0.001). We can say that these side effects can be expected and explained by mechanism of actions of drugs as dexmedetomidine inhibits central sympathetic outflow by blocking the presynaptic alpha receptors in the brainstem, thereby inhibiting the release of norepinephrine which causes bradycardia and hypotension in contrast to ketamine that acts as an antagonist at muscarinic and nicotinic acetylcholine receptors, blocks sodium and potassium channels, activates high-affinity D2 dopamine receptors and L-type voltage-gated calcium channels, and facilitates gamma- aminobutyric acid (GABA) inhibition increasing neurotransmitters such as norepinephrine, dopamine, and serotonin in the brain which stimulates the sympathetic nervous system resulting in tachycardia and hypertension which masks its direct cardiac depressant effects. These results are approved by Shehabi et al. in their study on dexmedetomidine who recorded more adverse events in the dexmedetomidine group than in the usual-care group, most commonly bradycardia and hypotension (0.7% versus 0.1%, respectively; P=0.003) [16] and Basagan-Mogol et al. study who examined hemodynamic response to ketamine in cardiac surgery patients and found that ketamine provided satisfactory hemodynamics during induction, but commonly led to tachycardia [36]. Concerning nausea and vomiting, we found a higher incidence of these effects in the control group. This side effect can be explained by the occurrence of more pain sensation and more use of opioids in the control group than in the other two groups. Regarding dexmedetomidine, Taghinia et al. reported that dexmedetomidine decreases narcotic use, thereby further improving respiratory safety and decreasing PONV decreasing antiemetic use [37]. Regarding ketamine, Groth et al. reported that risks of opioids include nausea and vomiting, constipation, ileus, immunosuppression, and delirium, and the use of ketamine as nonopioid pain medications can reduce opioid exposure reducing these risks [38]. Also, Piper et al. in their study on ketamine in adult cardiac patients reported that Ketamine administration in the intensive care unit decreased the incidence of shivering and PONV after cardiac surgery [35].
One limitation of this study was that all study populations were egyptians, limiting the generalization of the data for different races and ethnicities. Another limitation was the pain of our study was assessed through the VAS score. Consequently, the patients who developed postoperative respiratory failure, needed reintubation, experienced surgery-related complications, or needed re- intervention were excluded because of their reduced ability to answer the investigator's questions. Also, we tested this modality in the intensive care unit after cardiac surgery. So, a randomized controlled trial needs to test its effectiveness in its possibility to achieve opioid-free or opioid-less cardiac anesthesia between these beneficial drugs.
Conclusion
In conclusion, the use of either ketamine or dexmedetomidine is recommended in the postoperative period as an adjuvant modality and a good idea to decrease postoperative opioid use with their multiple side effects and improve patient satisfaction score in patients undergoing open heart surgery under general anesthesia with observation of possibility of some preventable side effects.
Ethics Approval and Consent to Participate
The ethical review board of Fayoum University Hospital approved the study design before the start of the study (D294) according to the relevant guidelines and regulations. Written informed consent was obtained from all patients.
References
- Gan Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J Pain Res. 2017; 10: 2287-2298.
- Chapman CR, Vierck The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. 2017; 359: 1-38.
- Wang Y, Sands LP, Vaurio L, et al. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007; 15: 50-59.
- Roberts GW, Bekker TB, Carlsen HH, et al. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related Anesth Analg. 2005; 101: 1343-1348.
- Mulier JP. Perioperative opioids aggravate obstructive breathing in sleep apnea syndrome: Mechanisms and alternative anesthesia strategies. Curr Opin Anaesthesiol. 2016; 29: 129-133.
- Lavand’homme P, Estebe JP. Opioid-free anesthesia: A different regard to anesthesia Curr Opin Anaesthesiol. 2018; 31: 556-561.
- Kehlet H, Joshi Enhanced recovery after surgery: current controversies and concerns. Anesth Analg. 2017; 125: 2154-2155.
- Joshi GP, Kehlet H. Enhanced recovery pathways: looking into the future. Anesth Analg. 2019; 128: 5-7.
- Bell R, Dahl J, Moore R, et al. Periâ?operative ketamine for acute postoperative pain: a quantitative and qualitative systematic review (Cochrane review). Acta Anaesthesiol Scand. 2005; 49: 1405-1428.
- Reves JG, Glass PSA, Lubarsky DA, et al. Intravenous anesthetics. Anesthesia. 2010; 719-768.
- Amer M, Maghrabi K, Bawazeer M, et Adjunctive ketamine for sedation in critically ill mechanically ventilated patients: an active-controlled, pilot, feasibility clinical trial. J intensive care. 2021; 9: 54.
- Reese J, Sullivan V, Boyer N, et al. Non-comparative prospective pilot study of ketamine for sedation in adult septic shock. Wilderness Environ Med. 2018; 29: 211-214.
- Mazzeffi M, Khelemsky Poststernotomy pain: A clinical review. J Cardiothorac Vasc Anesth. 2011; 25: 116378.
- Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: A contemporary and practical review. J Geriatr Cardiol. 2012; 9: 197208.
- Carollo DS, Nossaman BD, Ramadhyani Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008; 21: 457-461.
- Shehabi Y, Ruettimann U, Adamson H, et Dexmedetomidine infusion for more than 24 hours in critically ill patients: Sedative and cardiovascular effects. Intensive Care Med. 2004; 30: 2188-2196.
- Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit Am J Respir Crit Care Med. 2002; 166: 1338-1344.
- Roberts JS, Laughlin JE, Wedell DH. Validity issues in the Likert and Thurstone approach to attitude Educ Psychol Meas. 1999; 59: 211-233.
- Lahtinen P, Kokki H, Hakala T, et ketamine as an analgesic adjunct reduces opioid consumption after cardiac surgery. Anesth Analg. 2004; 99: 1295301.
- Carollo DS, Nossaman BD, Ramadhyani Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008; 21: 457-461.
- Schnabel A, Reichl SU, Zahn PK, et al. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain. 2013; 154: 1140-1149.
- Le Bot A, Michelet D, Hilly J, et Efficacy of intraoperative dexmedetomidine compared with placebo for surgery in adults: a meta-analysis of published studies. Minerva Anestesiol. 2015; 81: 1105-1107.
- Liu Y, Zhao G, Zang X, et Effect of dexmedetomidine on opioid consumption and pain control after laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. Wideochir Inne Tech Maloinwazyjne. 2021; 16: 491-500.
- Wieruszewski PM, Leung JG, Nelson S. Ketamine use in the intensive care AACN Adv Crit Care. 2018; 29: 101-106.
- Radvansky BM, Shah K, Parikh A, et al. Role of ketamine in acute postoperative pain management: a narrative review. Biomed Res Int. 2015; 2015: 749837.
- Patanwala AE, Martin JR, Erstad BL. Ketamine for analgosedation in the intensive care unit: a systematic J Intensive Care Med. 2017; 32: 387-395.
- Guillou N, Tanguy M, Seguin P, et al. The effects of small- dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003; 97: 843-847.
- Abowali HA, Paganini M, Enten G, et Critical review and meta-analysis of postoperative sedation after adult cardiac surgery: dexmedetomidine versus propofol. J Cardiothorac Vasc Anesth. 2021; 35: 1134-1142.
- Lewis K, Piticaru J, Chaudhuri D, et Safety and efficacy of dexmedetomidine in acutely ill adults requiring noninvasive ventilation: a systematic review and meta-analysis of randomized trials. Chest. 2021; 159: 2274-2288.
- Keating GM. Dexmedetomidine: A review of its use for sedation in the intensive care setting. Drugs. 2015; 75: 1119-
- Giovannitti Jr JA, Thoms SM, Crawford Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015; 62: 31-39.
- Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46: 825-
- Guillou N, Tanguy M, Seguin P, et al. The effects of small- dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003; 97: 843-847.
- Piper SN, Beschmann R, Mengistu A, et al. Assessment of recovery, dreaming, hemodynamic, and satisfaction in post- cardiac surgery patients receiving supplementary propofol sedation with S Minerva Anestesiol. 2009; 75: 363-373.
- Piper SN, Beschmann RB, Mengistu A, et al. Postoperative analgosedation with S ketamine decreases the incidences of postanesthetic shivering, nausea, and vomiting after cardiac surgery. Med Sci Monit. 2008; 14: 59-65.
- Basagan-Mogol E, Goren S, Korfali G, et Induction of anesthesia in coronary artery bypass graft surgery: The hemodynamic and analgesic effects of ketamine. Clinics (Sao Paulo). 2010; 65: 133-138.
- Taghinia AH, Shapiro FE, Slavin SA. Dexmedetomidine in aesthetic facial surgery: improving anesthetic safety and efficacy. Plast Reconstr Surg. 2008; 121: 269-276.
- Groth CM, Droege CA, Connor KA, et al. Multicenter Retrospective Review of Ketamine Use in the Crit Care Explor. 2022; 4: 0633.