Deep Abscess in Diabetic Patients
Author'(s): Lygie Sephora Kibhat Odiki, Nassim Essabah Haraj, Amal Mjabber, Siham El Aziz, and Asma Chadli*
Endocrinology, Diabetology and Metabolic Disease Department, Bn Rochd University Hospital. Neurosciences and Mental Health Laboratory. Faculty of Medicine and Pharmacy- University Hassan II Casablanca, Morocco.
*Correspondence:
Asma Chadli, Endocrinology and Diabetology Department, Ibn Rochd University Hospital of Casablanca, Tel: +212 661092820.
Received: 12 September 2020 Accepted: 04 October 2020
Citation: Odiki LSK, Haraj NE, Mjabber A, et al. Deep abscess in diabetic patients. Diabetes Complications. 2020; 4(3); 1-4.
Abstract
Introduction: Uncontrolled diabetic patients are susceptible to infections, especially deep abscesses. The objective of our work was to analyze, clinical, biological, radiological and bacteriological characteristics of deep abscesses in patients who have diabetes and identify the factors that promote them.
Patients and Methods: We included 120 diabetic patients hospitalized from June 2017 to November 2018.
Results: The characteristics of our patients revealed a sex ratio H/F of 1.55 with a mean age of 46.72 ± 15 years. Type 2 diabetes predominates (79.2%) and mean HBA1c was 10.54 ± 2%. Admitted patients with hyperglycemia (67.5%) and ketosis (22.5%), in a feverish context (44.2%). Clinical signs were localized pain depending on the site of infection in 45.8% of patients and 10% had atypical signs. Abscess sites were polymorphic. The main contributing factor to these infections was irregular follow-up (49%, p<0,001). Imaging guided the therapeutic conduct. Treatment consisted of intensified insulin therapy, surgical drainage, antibitherapy. The evolution was favorable in 97.5% and 3 patients died of post-operative pulmonary embolism or septic shock.
Conclusion: We found a high proportion of chronic hyperglycemia associated with deep abscesses, underscoring the value of careful clinical examination even when an unspecific clinical picture.
Keywords
Background
Diabetes mellitus is a clinical condition associated with a deficiency in the secretion or action of insulin. It is considered one of the greatest emerging health threats of the 21st century. It’s estimated that 700 million people in 2045 year will have diabetes compared, more than 463 million currently worldwide [1]. In addition to the classic complications of the disease, diabetes has been associated with a decrease in T cell response, neutrophil function and humoral immunity disorders [2-4]. As a result, diabetes increases susceptibility to infections, both the most common and those affecting unusual sites caused by rare germs [5-9]. Such infections can affect the patient's balance due to their severity and trigger complications related to the underlying pathology, such as high blood sugar and ketoacidosis. Hence early medico-surgical and multidisciplinary management allows for better results, in order to improve patients' prognosis [10].
The purpose of our work was to determine the clinical, paraclinical, etiological and progressive characteristics of deep abscesses in diabetic patients.
Patients and Methods
Type of study
We conducted a cross-sectional study, including 120 diabetic patients hospitalized in different departments of the Ibn Rochd University Hospital in Casablanca and presenting an abscess (bacterial, parasitic, mycotic...) during the period from June 2017 to November 2018.
Variables studied
An exploitation sheet report collected socio-demographic parameters (age, sex, residence, marital status, occupation, socio- economic level, social security coverage), history (taking steroids and non-steroidal anti-inflammatory drugs: NSAIDs), toxic habits (smoking, alcohol), diabetes history (diabetes duration, treatment regimen, treatment supply, compliance, acute and chronic complications), Glycated hemoglobin (HbA1c). An irregular follow-up was considered if her was a more than 6 months with no consultation control. Other parameters concerning the infection such as the abscess characteristics (seat, germ involved, entrance door), markers of clinical and biological inflammation (blood count, c-reactive protein and bacteriological sampling), radiological assessment (ultrasound and CT scan). Were collected finally, the indicated treatment such as antibiotic therapy and surgical drainage with evolution were noted.
Statistical analysis
All data were calculated using SPSS version 25 software and excel 2007 for descriptive statistics. A value of p < 0.05 was considered as statistically significant.
Results
Study population description
We included 120 patients, 61% male and 39% female patients with a sex ratio H/F of 1.55 with a mean age of 46.72 ± 15 years. Table 1 illustrates the study population characteristics.
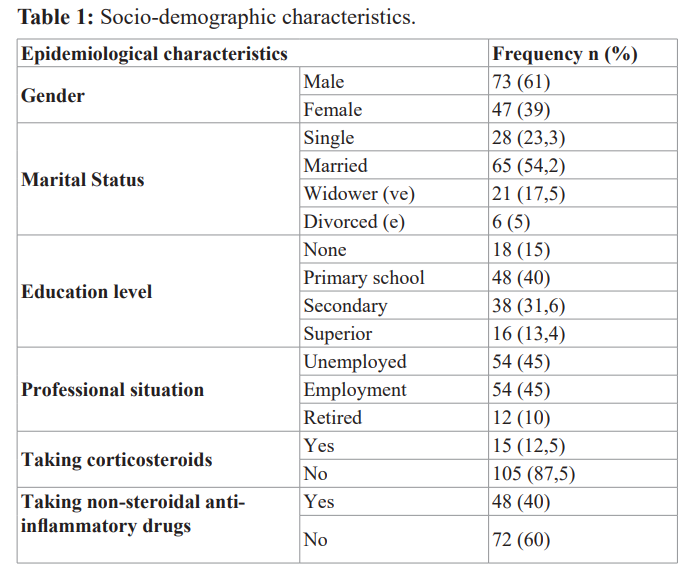
Characteristics of diabetics
We noted a predominance of type 2 diabetic patients in 79% of the study population and 21% of type 1 diabetic patients. Mean diabetes age was 6.2 ± 4.6 years, with an average HBA1c of 10.54 ± 2%. Patients were admitted for hyperglycemia 67.5% and 22.5% for diabetic ketosis. Patients with to controlled diabetes represented only 7.5% of the patient against 92.5% with an uncontrolled diabetes. We found a poor glycemic control in patients with type 2 diabetes in 72.5% of cases compared to 20% of patients with type 1 diabetes.
Factor leading essentially the irregularity of the follow-up in 49% of cases (p<0.001, r=0.08).
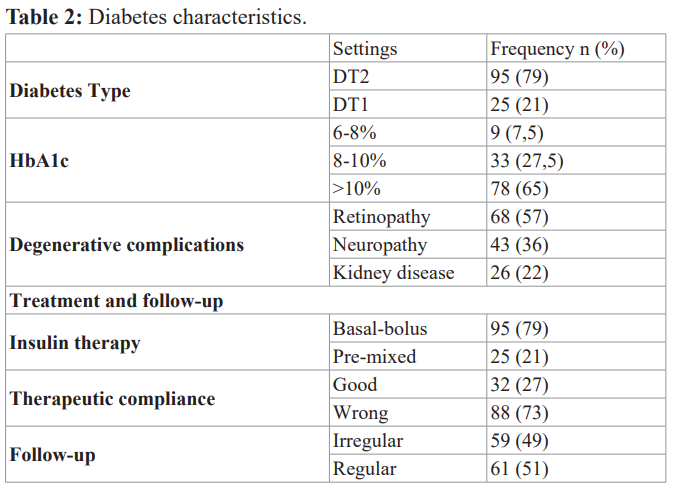
Infections Characteristics
The clinical signs found in our patients were fever in 44.2%, localized pain in 45.8% of cases and atypical pain in 10%. The entry door identified in only 5% of patients with deep skin abscesses. The abscesses found by localization were shown in Figure 1.
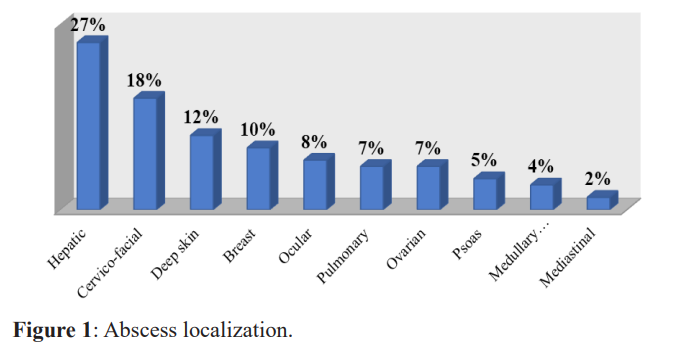
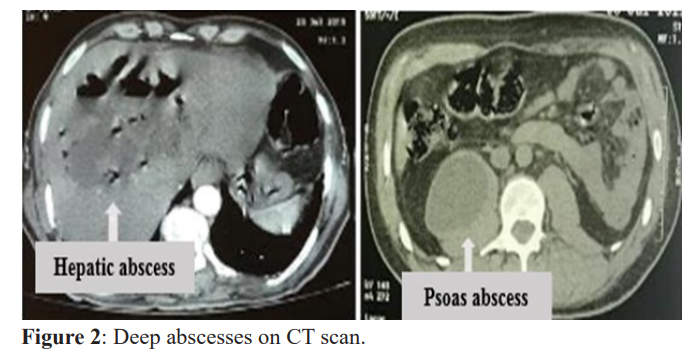
Overall, 69.16% of our patients had hyperleukocytosis, with an average of 13165.5 ± 5390 mg/l, predominantly neutrophil polynuclear, with an average of 8571.4 ± 2891.7 elements/mm3. An increase in C-reactive protein (CRP) was observed in 95% of patients with an average of 54.8 ± 73 mg/l. CT scan revealed variable lesions often associated with significant infiltration and/ or collection in 70%. Ultrasound objectified multiple abscesses in 59.2% of cases with an average size of seven centimeters. Figure 2 illustrates the deep cutaneous abscesses.
Bacteriological examination by blood culture in 34% and pus sampling in 66% isolated Escherichia. Coli in 28.3% of cases, Klebsiella pneumonia in 15.8% of cases mainly at the hepatic level, pseudomonas aeroginas in 10% of cases with a prevalence of corneal abscesses and a specificity of Aspergillus fumigatus in the patient with a complicated pan-sinusitis after a abscessed orbital cellulitis. Cocci gram positive, especially staphylococci and streptococci, in 25.8% of patients represented the second line of germs involved in abscesses.
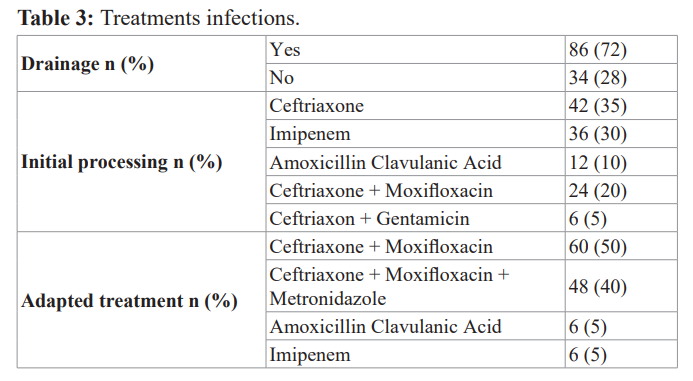
No bacteria where been isolated in 20% of our patients. Patients received drainage, single after double/ triple probabilistic intravenous antibiotherapy at baseline, then after adapted according to the antibiogram with an average treatment duration of 14 ± 7 days. The treatment characteristics of are illustrated in Table 3.
Discussion
Our study reported predominance of 61% a male against 39% of female, which is similar to the results of several studies [11,12]. The infections of patient diabetic did not differ in the both sexes, nor in the clinical presentation with the non-diabetic. With respect to history, the percentages of self-medication with non- steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids were similar to the findings of several studies, although NSAIDs accelerate the spread of infection and reduce inflammatory signs leading to late use of care [13]. 92.5% of patients were chronically with hyperglycemia, which explains the immunosuppression linked to the alteration of phagocytic functions of leukocytes that promote infection. Abscesses, characterized by pain in some cases as found in our study in 45.8% of patients, fever in 44.2% and atypical forms in others thus show the differentiation of clinical presentation according to the sites. Xaodong et al. [11] in a study on risk factors for fatal complications of maxillofacial infection found fever in 42.5%, pain in 96%, inconsistent with our study due to the specific location at the head level. On the other hand, for Wen-Jing wang et al. [14] found that fever was present in 91.6% consistent with our results.
The abscess sites identified on the basis of the clinic and radiology were done by Kalpana Sharma et al. [15] who found a rate of maxillofacial abscess in 29% of patients. As well as, Hong-Hau Wang et al. [16] a rate of 54.3% of liver abscesses in diabetic patients, this percentage can be explained by the fact that the study population was larger, including 221 patients. For Rossi et al. [17] the prevalence of liver abscesses was 21%, similar to our results. The study conducted by Jenkes et al. [12] in Colorado on 770 patients with skin abscesses showed a frequency of 33% of diabetic patients, almost similar to our results, although an entry door was found in only 5% of cases. The particularity in our study was the spinal cord injury determining the multifocality of infections, as identified by Noriyoshi Takebe et al. in one case [18]. Frequent liver abscesses in diabetic patients, the most common germs involved were E. coli in 18.1% of cases and streptococcus in 29.5%, followed by Klebsiella pneumoniae identical to our results and those found by Lin YT et al. [19-22].
Pseudomonas aeroginas has been isolated mainly in ocular abscesses. In case of cutaneous abscesses, a similar identified microorganism was cocci-positive (90%) [12]. Staphylococci and streptococci, the third most commonly reported bacteria, showed high sensitivity to commonly prescribed antibiotics.
The first attitude in our centers being a probabilistic spector antibiotic therapy, not allowing time for possible colonization by nosocomial flora, has been corrected not only according to the antibiotic susceptibility test but also, taking into account the pathology evolution [12].
In this study, gram-negative bacteria were sensitive to 3rd generation cephalosporin in combination with moxifloxacin. Therapeutic adjustment in antibiotic therapy was observed in 25% in dual therapy and 40% required triple antibiotic therapy. A study would have shown that high HBA1c was a prognostic factor in hospital mortality, requiring insulin therapy for patients receiving oral antidiabetic agents in our series and taking into account the recommendations to improve patients' vital prognosis [23-25].
Conclusion
We noted in our study that the often subtle or non-specific clinical presentation of potentially severe deep abscesses can lead to hyperglycemia and ketoacidosis in diabetic patients, with a predisposition to imbalance and/or poor therapeutic compliance. However, the bacteria polymorphism requires antibiotic susceptibility tests as soon as possible in order to obtain a better prognosis. Thus, management must be multidisciplinary, hence the obligation to place particular emphasis on prevention through therapeutic education and careful clinical examination of diabetic patients.
References
- https://www.diabetesatlas.org
- Farnsworth CW, Shehatou CT, Maynard R, et al. A humoral immune defect distinguishes the response to Staphylococcus aureus infections in mice with obesity and type 2 diabetes from that in mice with type 1 Infect Immun. 2015;can be of various locations. Discordant studies to our series 83: 2264-2274.
- Li JZ, Li JY, Wu TF, et al. Helicobacter pylori Infection Is Associated with Type 2 Diabetes Not Type 1 Diabetes An Updated Meta Analysis. Gastroenterol Res Pract. 2017; 2017:
- Asare Anane H, Botchey CPK, Ofori EK, et Altered immunoglobulins A and G in Ghanaian patients with type 2 diabetes. SAGE Open Med. 2018; 6.
- Suaya JA, Eisenberg DF, Fang C, et al. Skin and soft tissue infections and associated complications among commercially insured patients aged 0-64 years with and without diabetes in the S. PLoS One. 2013; 8: e60057.
- Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with Diabetes Care. 2003; 26: 510-513.
- Egi Acute glycemic control in diabetics. How sweet is oprimal Con Just as sweet as in nondiabetic is better. J Intensive Care. 2018; 6: 70.
- Egi M, Furushima N, Makino S, et al. Glycemic control in acute Korean J Anesthesiol. 2017; 70: 591-595.
- Maines E, Franceschi R, Cauvin V, et Iliopsoas abscess in adolescents with type 1 diabetes mellitus. Clin Case Rep. 2015; 3: 638-642.
- Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients A large multicenter observational prospective study of 606 episodes and analysis of the factors related to the response to PLOS ONE. 2018; 13: e0204036.
- Han X, An J, Zhang Y, et Risk Factors for Life-Threatening Complications of Maxillofacial Space Infection. J Craniofac Surg. 2016; 27: 385-390.
- Jenkins TC, Knepper BC, Jason Moore S, et Comparison of the microbiology and antibiotic treatment among diabetic and nondiabetic patients hospitalized for cellulitis or cutaneous abscess. J Hosp Med. 2014; 9: 788-794.
- Righini CA, Motto E, Ferretti G, et al. Cellulites cervicales extensives et médiastinite descendante nécrosante Diffuse cervical cellulites and descending necrotizing mediastinitis. Ann Otolaryngol Chir 2007; 124: 292-300.
- Wang WJ, Tao Z, Wu Etiology and clinical manifestations of bacterial liver abscess A study of 102 cases. Medicine Baltimore. 2018; 97: e12326.
- Sharma K, Das D, Joshi M, et Deep Neck Space Infections A Study in Diabetic Population in a Tertiary Care Centre. Indian J Otolaryngol Head Neck Surg. 2018; 70: 22-27.
- Wang HH, Tsai SH, Yu CY, et The association of haemoglobin A 1 C levels with the clinical and CT characteristics of Klebsiella pneumoniae liver abscesses in patients with diabetes mellitus. Eur Radiol. 2014; 24: 980-989.
- Rossi G, Lafont E, Gasperini L, et al. Abcès hépatiques Liver Rev Med Interne. 2016; 37: 827-833.
- Takebe N, Iwasaki K, Hashikata H, et Intramedullary spinal cord abscess and subsequent granuloma formation a rare complication of vertebral osteomyelitis detected by diffusion-weighted magnetic resonance imaging. Neurosurg Focus. 2014; 37: E12.
- Lederman ER, Crum Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005; 100: 322-331.
- Meddings L, Myers RP, Hubbard J, et A population- based study of pyogenic liver abscesses in the United States incidence mortality and temporal trends. Am J Gastroenterol. 2010; 105: 117-124.
- Fung CP, Chang FY, Lee SC, et al. A global emerging disease of Klebsiella pneumoniae liver abscess is serotype K1 an important factor for complicated endophthalmitis Gut. 2002; 50: 420-424.
- Lin YT, Wang FD, Wu PF, et al. Klebsiella pneumoniae liver abscess in diabetic patients association of glycemic control with the clinical BMC Infect Dis. 2013; 13: 56.
- Gornik I, Gornik O, GasparoviÄ? HbA1c is outcome predictor in diabetic patients with sepsis. Diabetes Res Clin Pract. 2007; 77: 120-125.
- Mahmoodpoor A, Hamishehkar H, Shadvar K, et Relationship between glycated hemoglobin Intensive Care Unit admission blood sugar and glucose control with ICU mortality in critically ill patients. Indian J Crit Care Med. 2016; 20: 67-71.
- American Diabetes Association. Diabetes Care in the Hospital Standards of Medical Care in Diabetes 2018. Diabetes Care. 2018; 41: S144-S151.