Development of Diabetes: Possible Role of Potassium
Author'(s): Tarun Saxena1*, Manjari Saxena2, Azeema Ozefa Ali3
1MD (Internal Medicine), Senior Consultant, Department of Internal Medicine, Mittal Hospital and Research Centre, Ajmer, India.
2M. SC., PGDYHS Department Yoga and Physical Education, Mittal Hospital and Research Centre, Ajmer, India.
3B.H.M.S. Resident Doctor, Department of Internal Medicine, Mittal Hospital and Research Centre, Ajmer, India.
*Correspondence:
Tarun Saxena, MD (Internal Medicine), Senior Consultant, Department of Internal Medicine, Mittal Hospital and Research Centre, Ajmer, India, Tel: +91-9829089284; Email: yogdiab@gmail.com and info@mittalhospital.com.
Received: 11 September 2017 Accepted: 03 October 2017
Citation: Tarun Saxena, Manjari Saxena, Azeema Ozefa Ali. Development of Diabetes: Possible Role of Potassium. Diabetes Complications. 2017; 1(3); 1-6.
Abstract
Background: Development of type-2 diabetes remains ambiguous. There are some known risk factors like stress, lack of exercise etc. are described in diabetes. How risk factors affect insulin kinetics? Therefore to assess the risk factors and their possible effect on insulin kinetics in the study population was the main focus of the study.
Methods: This is a descriptive study done in retrospective manner in Ajmer central part of Rajasthan, India based on data collection of admitted patients. Data of 200 cases of diabetes (group A) was compared with 200 non-diabetic (group B) cases for dietary intake, history of stress and exercise. Blood samples were examined for lactic acid, PH, potassium levels.
Results: In group A and group B no major difference was there in BMI and positive family history. Group A cases had low carbohydrate intake in diet, severe mental exhaustion/stress and poor exercise schedule. This was associated with low lactic acid, alkaline PH and hypokalemia in group A.
Conclusion: Poor exercise and low carbohydrate diet are associated with low lactic acid resulting in alkaline PH associated with hypokalemia. Stress is associated with increase in basal sympathetic discharge resulting in hypokalemia. Low serum potassium possibly inhibits glucose and potassium entry into muscle at its receptor and simultaneously reduces insulin release. Chronicity of such conditions result in development of diabetes.
Keywords
Introduction
The pathogenesis of type-2 diabetes remains ambiguous. Primary events leading to/precipitating type 2 diabetes are largely unclear. Various studies have documented multiple risk factors like genetic defect [1-5], dietary factors (high fat, less carbohydrate, less fibre diet [6-8], stress [9-14], obesity [15-18], and lack of physical activity [19], are responsible for diabetes. A few studies have found relation of serum K+ (potassium) [20] in diabetes. Therefore the study was aimed to review the contribution of all these risk factors and role of K+ affecting insulin kinetics, in developing diabetes. Additionally the study was focused upon any common end point related to all these risk factors in insulin kinetics.
Methods
This is a descriptive study done in retrospective manner in central part of Rajasthan, India, based on data collection of patients admitted in Mittal hospital and research centre, Pushkar road and Keshvam hospital Maharana Pratap Nagar Ajmer. Arbitrarily record of 200 freshly diagnosed cases of type-2 diabetes (incidental diagnosis) (group A) where no previous history of any treatment of diabetes was available, was compared with records of other 200 non- diabetic people (group B) admitted in Mittal hospital for some other diseases between July 2016 and July 2017. All 400 cases belong to urban population of Ajmer city and represent a small sample of same population (one million). Only those cases were selected where a complete dietary, exercise, stress history and following investigation records were available. The permission was taken from hospital ethical committee.
Data was examined for
- Basal characteristics including age, sex, BMI, vital parameters including pulse rate, blood pressure, temperature
- Dietary history, food habits
- History of physical exercise
- Mental health life style factors- duration of mental work, duration of sleep
- Stress
- Blood investigations including ABG (arterial blood gas
- analysis) including blood PH, serum sodium, serum potassium, serum calcium, serum lactic acid and blood glucose
- Serum urea, serum creatinine, glycosylated hemoglobin
- Significant past history
Blood investigations data used was selected only at the time of admission i.e. before commencement of any treatment. Blood glucose, serum K+, serum sodium and lactic acid levels were also measured in laboratory.
Exclusion- Diabetic ketoacidosis
Results
In group A and group B no major difference was there in BMI and positive family history. Group A cases had low carbohydrate intake in diet, severe mental exhaustion/stress and poor exercise schedule. This was associated with low lactic acid, alkaline PH and hypokalemia in group A (Tables 1,2,3,4 and ABG images 1-6).
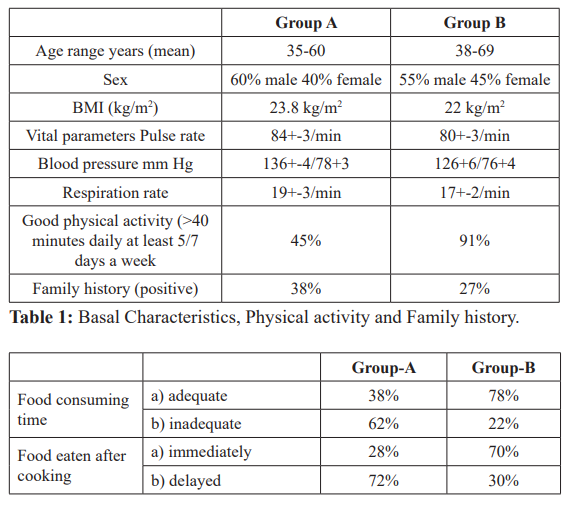
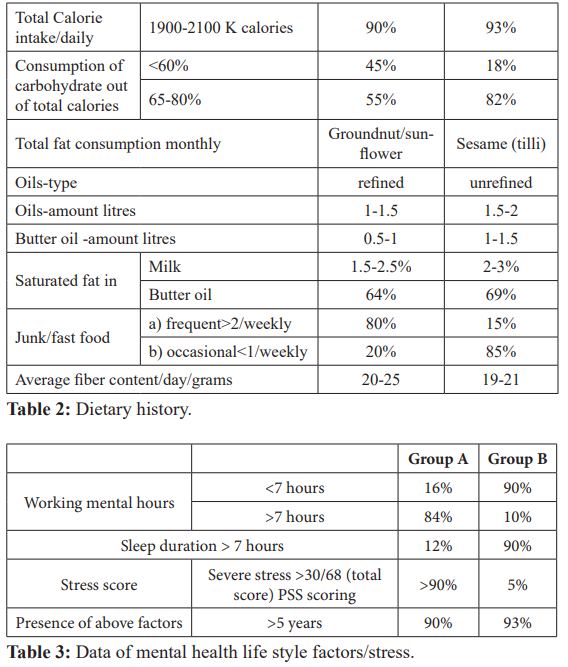
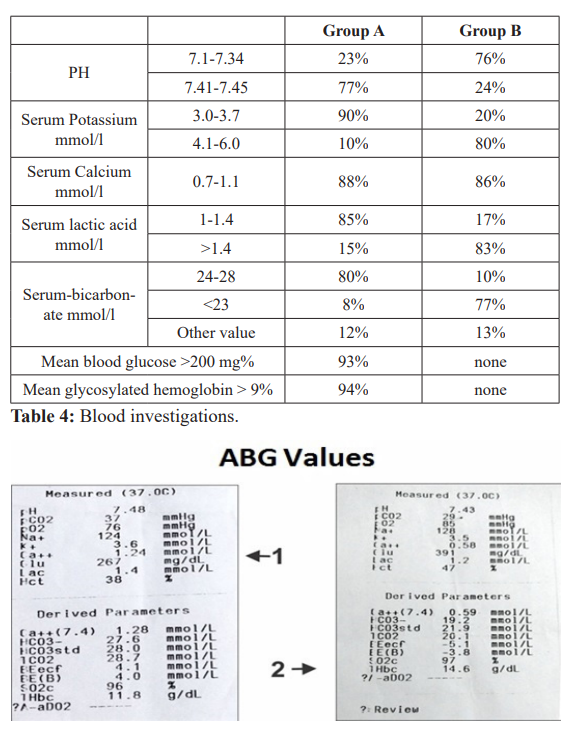
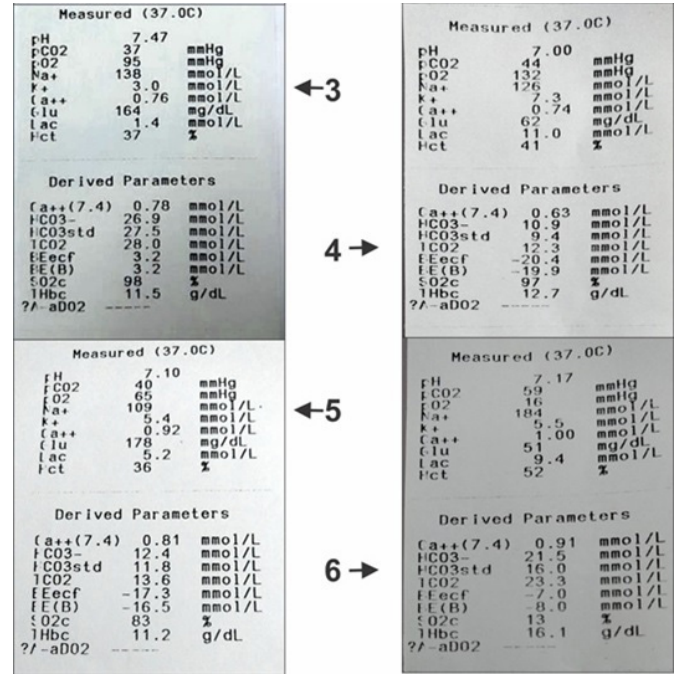
ABG Values- Arterial blood gas analysis.
ABG 1,2,3: Alkaline PH, low potassium, low lactic acid and high blood glucose.
ABG 4: Acidic PH (metabolic, low bicarbonate), high potassium, high lactic acid and low blood glucose.
ABG 5: Same case (4) after 50 ml of sodium bicarbonate slight increase in PH, bicarbonate and glucose, reduction in potassium.
ABG 6: Acidic PH (respiratory, high pco2), high potassium, high lactic acid and low blood glucose.
Discussion
Worldwide, the incidences of diabetes are on an extensive rise. Reduction in insulin secretion from pancreas and insulin resistance in liver, muscle, adipose tissues is the prime pathology responsible for diabetes. However; why there is insulin resistance and reduction in insulin secretion is not fully understood. Various modifiable risk factors associated with diabetes are obesity, lack of exercise, diet lacking in carbohydrates and fibres, mental exhaustion/ mental stress etc. chronicity of these factors; i.e. persistence for longer periods; increases the probability in a person to develop diabetes. Despite extensive evaluation exact effect of these risk factors in the etio-pathogenesis remains unexplained [8]. Prevention and holding the progression of disease remains important. The role of PH and serum K+ in insulin kinetics remains dubious. A few studies have depicted the role of K+ in diabetes. Still a clear relationship between risk factors, K+, insulin secretion, utilization and diabetes is not documented. Therefore, this study was planned to observe any correlation between various risk factors, blood K+ and diabetes. The study was done in a descriptive, retrospective manner through data collection in Mittal hospital and research centre, Pushkar road and Keshvam hospital Maharana Pratap Nagar Ajmer, Rajasthan, India. Hospital record of 400 cases (200 diabetic (study group group A) and 200 non diabetic control group group B) admitted between July 2016 and July 2017 was observed (only those cases where a detailed history of diet and stress was available).
We divide the discussion in four parts Primary findings related to basal characteristics and risk factors
There was no major difference in BMI, positive family history and vital parameters in both groups (Table 1). There was no overt increase in pulse rate (sympathetic activity) and respiratory rate in either group.
In dietary history in group A v/s B group there was major difference in total carbohydrate intake 55 v/s 82%, fast food intake 80% v/s 20% and time given in consuming food. (Group A v/s group B, table 2) i.e. less carbohydrate, high fast food diet with hasty eating in patients with high blood glucose. No gross difference in fat consumption, fibre consumption in either group.
In group A v/s group B there was major difference in exercise duration and regular schedule table 1. Group A cases had poor exercise schedule as compared to group B.
Assessment of mental health life style factors suggested a state of more mental work and less mental rest in group A as compared to group B (84% v/s 12%). In group A even the duration of sleep was less (12% v/s 90%). Group A Cases were severely stressed based on PSS (93% V/S 10%). There was chronicity in such life style factors i.e. in > 90% present for at least 5 years (Table 3).
Over all up to 80% of cases had combination of at least 2 risk factors including diet/exercise and mental exhaustion/stress i.e. Less carbohydrate diet consumed quickly, poor exercise and mental exhaustion associated with diabetes.
Secondary findings related to blood investigations and their association with risk factors
A.Secondary findings- In group A cases PH was towards alkaline side as compared to group B (7.41-7.45 v/s 7.1-7.34). Serum K+ level were at lower side in group A as compared to group B (3.0- 3.7 v/s 4.1-6). Serum lactic acid was also at a lower side in group A v/s group B. (table 4) Presence of alkalosis, hypokalemia, low lactic acid and high blood glucose in group A (diabetic cases). (ABG values 1, 2, 3). On the contrary in group B acidic PH, high K+ and high lactic acid was associated with low blood glucose. (ABG value 4 and 6). ABG 5 is of the same case of ABG 4.
ABG 5 shows slight increase in PH after 50 ml of injection sodium bicarbonate associated with reduction in lactic acid, K+ and increase in blood glucose.
B.Association of secondary findings to risk factors (primary findings)? How In previous studies high carbohydrate diet specially made from wheat flour, freshly cooked, if consumed slowly generates high lactic acid in blood [21,22]. On the contrary diet like fast food or junk food, Chinese food consumed hurriedly provides less lactic acid. These findings are similar to low serum lactic acid levels found in group A and high lactic acid levels found in group B. In group A total carbohydrate consumption was < 50% in half a number of cases, less eating time and excess fast food consumption.
Good exercise generates good lactic acid and increases serum K+ levels [21-23], in muscles and vice versa. In group A lack of exercise is associated with low serum lactic acid and less serum K+ values.
Chronic stress and mental exhaustion is associated with increased baseline sympathetic discharge (There is physiological depletion of neurotransmitters and desynchronized activity of brain in stress and mental exhaustion associated with fast mental speed). This is depicted as loss of amplitude and gives low voltage fast Beta activity in EEG. This brain activity is associated with poor control of cortical areas to hypothalamus and there is increase in basal sympathetic discharge. Spikes are seen in SSR (Sympathetic skin response) assessment [9,24-29]. In group A there was chronic stress and mental exhaustion.
How secondary findings related to serum K+?
a.Low lactic acid is associated with alkaline PH which shifts extracellular K+ to intracellular space and results in hypokalemia [22], which is present in our findings in group A and conversely in group B [22]. Finding of ABG 4 and ABG 5 clearly indicates that increase in PH shifts K+ intracellular reduces insulin functioning associated with increase in blood glucose.
b.Increase in sympathetic activity is associated with persistent hypokalemia due to increase in activity of Na/K ATPase pump which shifts K+ intracellular, also present in current study in group A [21,22].
We summarize the findings
In group A there is less carbohydrate in diet, lack of exercise, chronic mental exhaustion/stress associated with alkaline PH, low serum lactic acid levels and hypokalemia. Low serum lactic acid results in alkaline PH and shifts extra cellular K+ to intracellular space (ECF to ICF). Increased sympathetic activity directly produces hypokalemia. Chronically hypokalemia affects insulin secretion and utilization and results in diabetes (findings in group A).
How hypokalemia affects insulin action. The possible mechanism seems to be following
A.Insulin secretory mechanism- Normally glucose entry in beta cells occurs through GLUT transporters, after entry glucose is metabolized, increases cellular ATP which inhibits K+ entry into cell through KATP channel. Low intracellular K+ results in depolarization of Ca++ (calcium) dependent exocytosis of stored insulin. There is increase in blood levels of insulin [31].
All those conditions which inhibit insulin release are associated with shift of K+ into cells. These include presence of insulin, Catecholamines /increased sympathetic activity, alkalemia, low lactic acids etc. [30-33]. These conditions are present in our study (group A) (Figure 1).
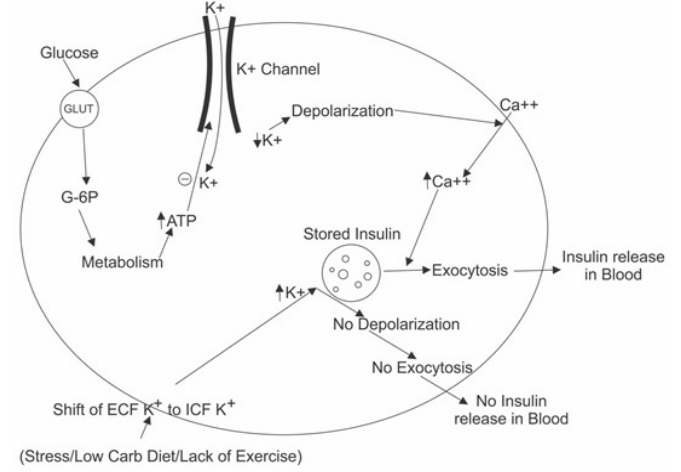
Figure 1: Insulin Release-Beta Cells of Pancreas.
B.Insulin utilization/sensitivity- This mechanism is difficult to understand but there are following steps in insulin utilization- After release insulin binds to its receptors (tyrosine kinase) in various tissues like muscles, adipose tissue and After binding to receptors there are various intracellular biochemical changes [31]. These lead to GLUT 4 (glucose transporter) expression over cells. This GLUT 4 expression allows entry of glucose inside the cells through facilitated diffusion.
When insulin concentrations are low, GLUT4 glucose transporters remains in a stored form and are not available for glucose transport. Presence of insulin is mandatory for their action. In some way entry of glucose through GLUT 4 is associated with K+ entry into the cells [31,32] (Figure 2).
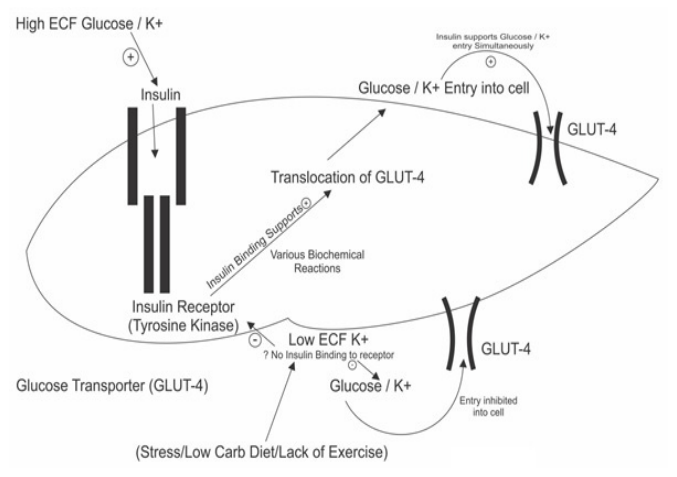
Figure 2: Insulin Utilization in Muscle.
Normally after food intake there is increase in blood glucose levels which shifts K+ out of the cells, reduces ICF K+ (ICF to ECF) increases ECF K+. High ECF and low ICF (especially Beta cells of pancreas) K+ quickly releases insulin in blood. This Insulin gets attached to its receptors in muscles fat tissue etc. and quickly shifts the K+ back into the cells associated glucose entry inside the cells.
Conditions which favour such physiology are good exercise, increase carbohydrate diet, stress/exhaustion free mind; all help in increase/ shift of ICF to ECF/blood K+ [32-34] (Figure 3).
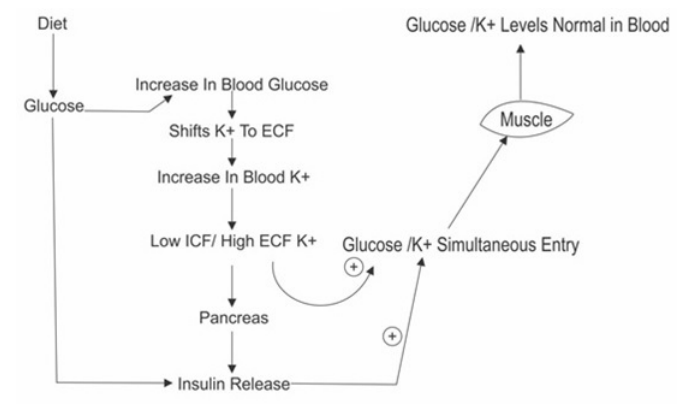
Figure 3: Normal Physiology (Insulin Kinetics).
Glucose entry through GLUT-4 transporters possibly involves simultaneous uptake of glucose and K+. ECF hypokalemia produces a resistant state where both insulin stimulated K+ uptake as well as insulin stimulated glucose uptake appears to be suppressed. It seems that, if hypokalemia is present then insulin release and utilization are suppressed.
Conditions opposing normal physiology are poor exercise, poor carbohydrate diet, increase mental stress/exhaustion; all result in less ECF/blood K+/ hypokalemia (high ICF). Thus inhibit insulin release and K+ intake into the cells associated with poor glucose uptake by cells and resulting in increase in blood glucose levels, if such risk factors prevail for years then. Disturb insulin releasing and utilization permanently and diabetes develops.
Various previous studies/references supporting role of K+ in insulin utilization are following
Exercise directly increases ECF K+ associated with increased sensitivity of insulin. Exercise also increases serum lactic acid levels which further shifts K+ from ICF to ECF [32,33].
Diuretics which decrease serum K+, decrease insulin sensitivity and increase risk of diabetes [20,35-37].
Diet low in K+ decreases insulin sensitivity [20,38,39]. Angiotensin system decreases serum K+ levels associated with decreased insulin sensitivity [20,40-42].
Hypothesis of the study: Development of Diabetes
Chronicity of mental exhaustion/stress along with lack of exercise/ poor carbohydrate diet leads to persistent hypokalemia (shift of ICF K+ to ECF K+) which possibly initially inhibits K+ /glucose entry into muscles/fat tissue. Later on hypokalemia affects insulin release. Lack of insulin further increases resistance non expression of GLUT- 4 transporters and persistence of such situation for many years’ results in diabetes.
Future projections
A prospective trial is required with a possible radioisotope attaching to K+ so as to understand the role of K+ in insulin kinetics in a better way.
Clinical Implications
Based on evidences of the study following can be done for diabetes prevention/management
- Correct primary risk factor mental exhaustion, diet and exercise to prevent hypokalemia.
a.Practice relaxation techniques to prevent burn out/ mental exhaustion/stress.
b.Sound sleep to restore neurotransmitters.
c.Adequate balance between mental work and rest.
d.Spending some time in garden to produce calmness in mind.
- Diet rich in carbohydrates to produce more lactic acid and fruits, lemon water, buttermilk directly producing acidic PH help in shifting ICF K+ to ECF K+ for better functioning of insulin.
- Proper exercise to improve insulin sensitivity
Acknowledgment
Bharat Saxena (figures) , Dr. Rajesh Sharma (biostat).
References
- Cook JTE, Hattersley AT, Levy JC, et al. Distribution of Type II diabetes in nuclear Diabetes. 1993; 42: 106-112.
- Knowler WC, Pettitt DJ, Saad M, et al. Diabetes mellitus in the Pina Indians: incidence, risk factors and pathogenesis. Diabetes/Metabo1ism 1990; 6: 1-27.
- Kaprio J, Tuomilehto J, Koskenvuo M, et Concordance for Type 1 (insulin dependent) and Type 2 (non-insulin- dependent) diabetes mellitus in population based cohort of twins in Finland. Diabetologia. 1992; 35: 1060-1067.
- Hattersley A, Turner R, Permutt M, et Linkage of type 2 diabetes to the glucokinase gene. Lancet. 1992; 339: 1307- 1310.
- Vionnet N, Stoffel M, Takede J, et al. Nonsense mutation in the glucokinase gene Causes early-onset non-insulin- dependent diabetes Nature. 1992; 356: 721-722.
- Marshall JA, Bessesen DH, Hamman RF. High saturated fat and low starch and fibre are associated with hyperinsulinaemia in a non-diabetic population: the San Luis Valley Diabetes Diabetologia. 1997; 40: 430-438.
- Marshall JA, Hamman RF, Baxter High-fat, low- carbohydrate diet and the etiology of non-insulin- dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol. 1991; 134: 590-603.
- Marshall JA, Hoag S, Shetterly S, et al. Dietary fat predicts conversion from impaired glucose tolerance to NIDDM: the San Luis Valley Diabetes Diabetes Care. 1994; 17: 50- 56.
- Saxena T K, Maheshwari S, Saxena Aetiopathogenesis of Type – 2 Diabetes Mellitus: Could Chronic Stress Play an Important Role? J Assoc physicians India. 2014; 62: 484-489.
- Wood Permanent Stress Linked to Type 2 Diabetes in Men. Psych Central. 2013.
- Mohan V, Deepa M, Anjana RM, et al. Incidence of Diabetes and Pre-diabetes in a Selected Urban South Indian Population (Cups - 19). Journal of Association of Physicians of India. 2008; 56: 152-157.
- Sleep and Type 2 diabetes mellitus. Clinical implications. S Ramnathan Iyer; J Assoc physicians 2012; 60: 42-46J.
- Askerstedt Psychosocial stress and impaired sleep. Scandinavian journal of work, environment & health. 2006; 36: 493-501.
- Marcora SM, Staiano W, Manning V. Mental fatigue impairs physical performance in Journal of Applied Physiology. 2009; 106: 857–864.
- Wilson PW, McGee DL, Kannel WB. Obesity, very low density lipoproteins and glucose intolerance over fourteen years: the Framingham study. Am J Epidemiol. 1981; 114: 697-704.
- Knowler WC, Pettitt DJ, Saad MF, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. Am J Clin 1991; 53: 1543-1551.
- Mckeigne PM, Pierpoint T, Ferrie VE, et al. Relationship of glucose intolerance and hyperinsulinaemia to body fat pattern in south Asians and Europeans. Diabetologia. 1992; 35: 785-
- Joffe BI, Panz VR, Wing JR, et al. Pathogenesis of noninsulin- dependent diabetes mellitus in the black population of southern Lancet. 1992; 340: 460-462.
- Fakhri 0, Fadhli AA, El Kawi RM, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Diabetes Care. 1997; 20: 537-544.
- Ranee Chatterjee, Hsin-chieh Yeh, David Edelman, et al. K+ and risk of type-2 diabetes. Expert Rev Endocrinol Metab. 2011; 6: 665-672.
- https://www.ncbi.nhm.gov/publed/7830383
- http://link.pringer.com.content/pdf110.1007
- Hutchinson RG, Barksdale B, Watson The effects on exercise on serum K+ levels. Chest. 1992; 101: 398-400.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived Journal of Health and Social Behavior. 1983; 24: 386-396.
- Vertugrono R, Liguori R, Corteglli P, et Sympathetic skin response: CliniCal autonomic research. 2003; 13: 256-270.
- Shani BT, helperin JJ, Boulu P, et Sympathetic skin response a method of assessment of unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry. 1984; 47: 536-542.
- Salvador M Guinjoan, Juan L Bemabo, Daniel P Cardinali. Cardiovascular tests of autonomic function and sympathetic skin responses in patients with major depression; Journal of Neurology, Neurosurgery, and 1995; 59: 299-302.
- intechopen-com/download/pdf/1098
- Wolfgang Klimesch. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews. 29: 169-195.
- https://www.ncbi.nim.nih.gov/books/NBK307/
- Westfall TC, Westfall DP. Neurotransmission: the autonomic and somatic motor nervous In: Goodman and Gilman’s. The pharmacological basis of therapeutics. 12th edn. New York: Mcgraw Hill. 2011; 1239-1243.
- Youn JH, Mcdonough Recent Advances in Understanding Integrative Control of K+ Homeostasis. Annu Rev Physiol. 2009; 71: 381-401.
- Peter S, Aronson, Gerhard giebisch. Effects of PH on K+: New explanation for old observations. J Am Soc Nephrol. 2011; 22: 1981-1989.
- Nenad K, Rosic, Mary L. et al. The Mechanism of Insulin stimulation of (Na+, K+) ATPase Transport Activity in The Journal of Biological Chemistry. 1985; 260: 6206-6212.
- Carter BL, Einhorn PT, Brands M, et al. Thiazide induced dysglycemia: Call for research from a working group from tha National Heart, Lungs, and Blood Hypertension. 2008; 52: 30-36.
- Zillich AJ, Garg J, Basu S, et Thiazide diuretics, K+, and the development of diabetes: a quantitative review. Hypertension. 2006; 48: 219-224.
- Rapoport MI, Hurd Thiazide-induced glucose intolerance treated with K+. Arch Intern Med. 1964; 113: 405-408.
- Chatterjee R, Yeh HC, Shafi T, et al. Serum and dietary K+ and risk of incident Type 2 diabetes mellius: the Atherosclearosis Risk in communities (ARIC) study. Arch Intern Med. 2010; 170: 1745-1751.
- Heianza Y, Hara S, Arase Y, et Low serum K+ levels and risk off type2 diabetes: the toranomon Hospital Health Management Center Study 1 (TOPICS1) Diabetologia. 2011; 54: 762-766.