Drug-coated Balloon Angioplasty Versus Drug-eluting Stenting for Femoropopliteal Arterial Disease: A Review of the Current Status
Author(s): Doo Sun Sim, MD, PhD1,2, Myung Ho Jeong, MD, PhD1 , Youngkeun Ahn, MD, PhD1 , Carlos MenaHurtado, MD, PhD3 , John Martin, MD, PhD4 , and Anthony Mathur MD, PhD2,5*
1 Department of Cardiovascular Medicine, Chonnam National University Hospital, Chonnam National University School of Medicine, Gwangju, Republic of Korea.
2 Centre for Cardiovascular Medicine and Devices, William Harvey Research Institute, Queen Mary University of London, London, UK.
3 Yale Vascular Outcomes Program, Section of Cardiovascular Medicine, Department of Internal Medicine, Yale University, Connecticut, USA.
4 Division of Medicine, University College London, London, UK.
5 Department of Cardiology, Barts Heart Centre, Barts Health NHS Trust, London, UK.
*Correspondence:
Anthony Mathur, Centre for Cardiovascular Medicine and Devices, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London, EC1M 6BQ. Tel: 0044 20 3765 8740.
Received: 02 Jan 2021; Accepted: 27 Jan 2022; Published: 31 Jan 2022
Citation: Doo Sun Sim, Myung Ho Jeong, Youngkeun Ahn, et al. Drug-coated Balloon Angioplasty Versus Drug-eluting Stenting for Femoropopliteal Arterial Disease: A Review of the Current Status. Cardiol Vasc Res. 2022; 6(1): 1-9.
Abstract
Endovascular procedures are frequently performed for symptomatic femoropopliteal disease. Drug-eluting stents (DES) and drug-coated balloons (DCB) were introduced to improve long-term outcomes and demonstrated superior outcomes to percutaneous transluminal angioplasty in randomised clinical trials. Femoropopliteal disease, however, can be challenging to treat using an endovascular approach as this segment suffers increased biomechanical stress during extremity movements, which may lead to chronic vascular injury or even stent fracture. The advantages of DCB include the direct and homogeneous delivery of an antiproliferative agent to the arterial wall, and the ability to reach tortuous and longer lesions without a vascular implant; however, the lack of scaffold makes the intervention prone to significant recoil. Even though the use of DCB, a leave-nothing-behind strategy, may appear desirable, the need for bailout stenting will increase as lesions become more complex. This review summarises and compares the currently available evidence regarding the use of DCB and DES in the treatment of femoropopliteal disease.
Keywords
Introduction
Atherosclerotic peripheral arterial disease (PAD) commonly involves the superficial femoral artery (SFA) and the popliteal artery (PA), and it is becoming increasingly prevalent [1]. Endovascular procedures are widely accepted as the first line of treatment for symptomatic PAD [2]. The 2017 European Society of Cardiology and European Society for Vascular Surgery guidelines on the treatment of PAD recommend an endovascular treatment as a class I indication in symptomatic femoropopliteal PAD with short (<25 cm) lesions [3], whereas the 2016 American College of Cardiology/American Heart Association guidelines give a class IIa recommendation for endovascular therapy in patients with femoropopliteal disease [4].
Percutaneous transluminalangioplasty (PTA) inthe femoropopliteal segment is associated with high rates of restenosis ranging from 40% to 50% within 1 year [5], limiting its use to very short lesions [6]. Previous trials using self-expanding, flexible nitinol stents suggested that stenting is more beneficial in longer femoropopliteal segments, compared to PTA alone [6-8]. However, bare-metal stents (BMS) were still associated with 30% to 40% restenosis within 2 to 3 years of implantation due to neointimal hyperplasia, causing in-stent restenosis (ISR) [9]. In order to overcome the limitations of both PTA and BMS, drug-based strategies such as drug-eluting stents (DES) and drug-coated balloons (DCB) were introduced to improve long-term outcomes [10]. DES and DCB have both demonstrated superior outcomes to PTA alone in randomised clinical trials (RCTs) [5,11-18]. Femoropopliteal disease, however, can be challenging to treat using an endovascular approach as this segment suffers increased biomechanical stress from repetitive deformations during extremity movements, which may lead to chronic vascular injury or even stent fracture [19]. The advantages of DCB include the direct and homogeneous delivery of an antiproliferative agent to the arterial wall without a vascular implant, and the ability to reach tortuous and longer lesions that would otherwise require multiple overlapping stents [20]. On the other hand, the lack of scaffold makes the intervention prone to significant recoil. This review summarises and compares the currently available evidence regarding the use of DCB and DES for the treatment of femoropopliteal disease.
Clinical Trials Comparing Drug-Based Strategies and PTA DES versus PTA
Table 1 details the DES trials for femoropopliteal disease included in this review. Currently, two self-expanding paclitaxel DES have received CE mark approval for use in patients with femoropopliteal disease. The Zilver PTX (Cook Corporation, Bloomington, IN, USA) is a nitinol self-expanding polymer-free stent coated with paclitaxel. In the Zilver PTX (Evaluation of the Zilver PTX Drug-Eluting Stent in the Above the-Knee Femoropopliteal Artery) RCT, SFA lesions treated with the Zilver PTX stent had a superior 12-month patency rate compared to PTA (83.1% vs

BMS, bare-metal stent; CD-TLR, clinically driven target lesion revascularisation; DES, drug-eluting stent; ISR, in-stent restenosis; MAE, major adverse event rate (defined as all causes of death through 1 month, major amputation of the target limb through 12 months, or CD-TLR through 12 months); PA, popliteal artery; PP, primary patency; PVDF-HFP, poly(vinylidene fluoride-co-hexafluoropropylene); PTA, percutaneous transluminal angioplasty; TLR, target lesion revascularisation.
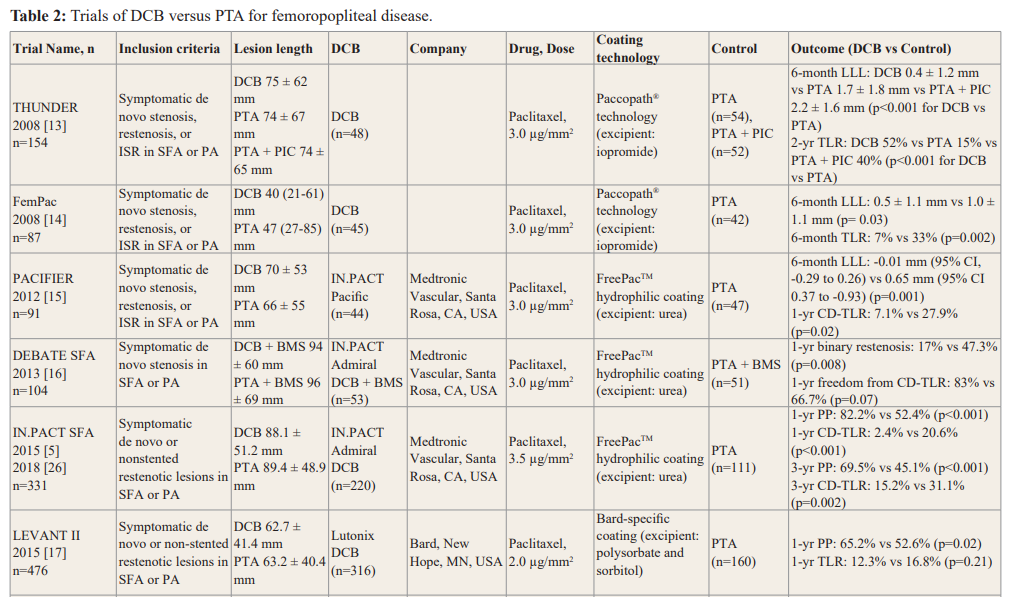
CD-TLR, clinically driven target lesion revascularisation; CI, confidence interval; DCB, drug-coated balloon; EU RCT, European Randomised Clinical Trial; ISR, in-stent restenosis; LLL, late lumen loss; PA, popliteal artery; PIC, paclitaxel in contrast medium; PP, primary patency; PTA, percutaneous transluminal angioplasty; SFA, superficial femoral artery; TLR, target lesion revascularisation.
32.8%, p<0.001) [11], and revealed sustained patency (66.4% vs 43.4%, p<0.01) and higher freedom from clinically driven target lesion revascularisation (CD-TLR) (83.1% vs 67.6%, p<0.01) at 5 years, compared to PTA [12]. The Eluvia (Boston Scientific, Marlborough, MA, USA) is a new polymer-coated, paclitaxel- eluting stent. The MAJESTIC (Stenting of the Superficial Femoral and/or Proximal Popliteal Artery Project) trial demonstrated the efficacy and safety of the Eluvia stent up to 3 years with primary patency of 96.4% at 1 year [21] and freedom from target lesion revascularisation (TLR) of 85.3% at 3 years [22]. In the IMPERIAL (ELUVIA Drug-eluting Stent Versus Zilver PTX Stent) RCT, the Eluvia stent showed non-inferior results to the Zilver PTX stent in primary patency and major adverse events at 12 months [23].
DES vs BMS
Data comparing DES and BMS for femoropopliteal lesions is scarce and conflicting (Table 1). The SIROCCO (Sirolimus Coated Cordis Nitinol Self-Expandable Stent for Treatment of Obstructive Superficial Femoral Artery Disease) trial did not demonstrate better results with sirolimus-eluting stents compared to BMS [24]. In the Zilver PTX trial, patients who were randomised to PTA and had suboptimal results were then randomised to provisional DES vs BMS. The DES group had improved 5-year primary patency and freedom from CD-TLR [12]. In contrast, in the recent BATTLE (Bare Metal Stent Versus Paclitaxel Eluting Stent in the Setting of Primary Stenting of Intermediate Length Femoropopliteal Lesions) RCT, the Zilver PTX DES failed to prove superiority over BMS in freedom from ISR at 1 year or in primary patency or TLR through 2 years [25].
DCB versus PTA
Clinical trials comparing DCB and PTA for femoropopliteal disease are summarised in Table 2. Early RCTs, THUNDER (Local Taxane with Short Exposure for Reduction of Restenosis in Distal Arteries), FemPac (Femoral Paclitaxel), and PACIFIER (Paclitaxel-coated Balloons in Femoral Indication to Defeat Restenosis) showed superiority of DCB over PTA in femoropopliteal disease [13-15].
In the IN.PACT SFA (Randomised Trial of IN.PACT Admiral Drug Coated Balloon vs Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) study, IN.PACT Admiral DCB (Medtronic Vascular, Santa Rosa, CA, USA) significantly improved 12-month primary patency compared to PTA (82.2% vs 52.4%, p<0.001), with sustained benefit at 3 years (69.5% vs 45.1%, p<0.001) [5,26]. The DEBATE-SFA (Drug Eluting
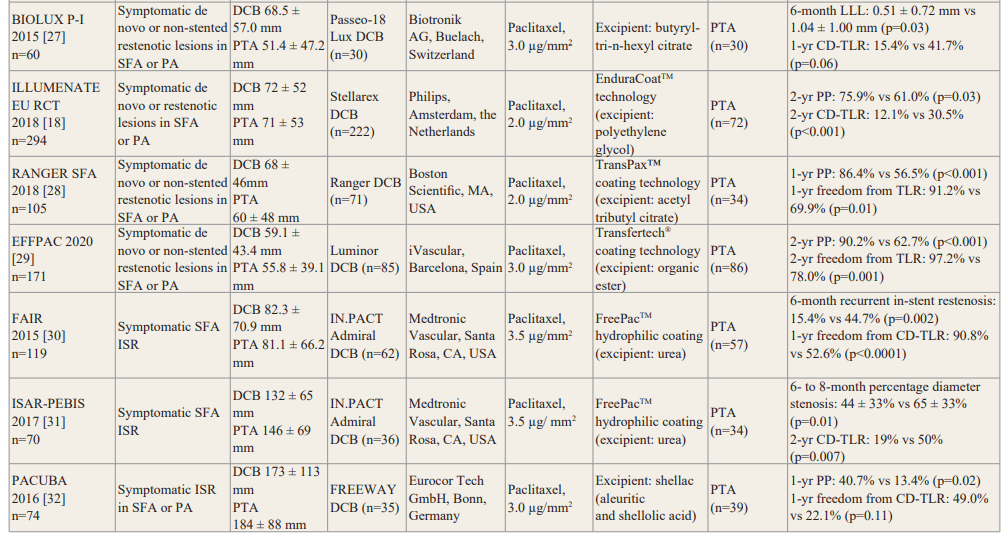
Balloon in Peripheral Intervention for the Superficial Femoral Artery) trial evaluated a strategy using DCB predilation followed by BMS placement. The use of IN.PACT Admiral DCB prior to BMS implantation was associated with lower restenosis (17% vs 47.3%, p=0.008) and higher rates of freedom from CD-TLR (83% vs 66.7%, p=0.07) compared to PTA prior to BMS at 12 months [16]. In the LEVANT 2 (Lutonix Paclitaxel-Coated Balloon for the Prevention of Femoropopliteal Restenosis) study, the use of Lutonix DCB (Bard, New Hope, Minnesota, USA) was associated with significantly improved 12-month primary patency (65.2% vs 52.6%, p=0.02), compared with PTA [17]. BIOLUX P-I (A Prospective, Multi-center, Randomised Controlled, First in Man Study to Assess the Safety and Performance of the Passeo-18 Lux Paclitaxel Releasing PTA Balloon Catheter vs the Uncoated Passeo-18 Balloon Catheter in Patients with Stenosis and Occlusion of the Femoropopliteal Arteries) evaluated the efficacy of the Passeo-18 Lux DCB (Biotronik AG, Buelach, Switzerland) in comparison with PTA in 60 patients. Late lumen loss at 6 months (0.51 ± 0.72 mm vs 1.04 ± 1.00 mm, p=0.03) and CD- TLR at 12 months (15.4% vs 41.7%, p=0.06) were lower in DCB group [27]. In the ILLUMENATE European Randomised Clinical Trial, the Stellarex DCB (Philips, Amsterdam, the Netherlands), composed of a low dose of paclitaxel (2 mg/mm2), was compared to PTA in 294 patients with femoropopliteal disease [18]. The DCB group showed higher primary patency at 2 years (75.9% vs 61.0%, p=0.03) and a lower rate of CD-TLR, compared with the PTA group (12.1% vs 30.5%, p<0.001). In the RANGER SFA trial, the Ranger DCB (Boston Scientific, MA, USA) showed higher primary patency (86.4% vs 56.5%, p<0.001) and freedom from TLR (91.2% vs 69.9%, p=0.01) at 1 year, compared to PTA [28]. Similarly, in the EFFPAC trial, the Luminor DCB (iVascular, Barcelona, Spain) was associated with higher primary patency (90.2% vs 62.7%, p<0.001) and freedom from TLR (97.2% vs 78%, p=0.001) at 2-year follow-up [29].
Several RCTs explored the outcome of DCB for the treatment of femoropopliteal ISR [30-32]. The FAIR (Femoral Artery In-Stent Restenosis) study randomised 119 patients with symptomatic SFA ISR to either IN.PACT Admiral DCB or PTA. The primary endpoint of recurrent ISR at 6 months was significantly lower in the DCB group compared to the PTA group (15.4% vs 44.7%, p=0.002). Freedom from TLR was higher with DCB compared to PTA at 12 months (90.8% vs 52.6%, p<0.001) [30]. The ISAR- PEBIS (Paclitaxel-Eluting Balloon Versus Conventional Balloon Angioplasty for In-Stent Restenosis of Superficial Femoral Artery) trial compared angioplasty using IN.PACT Admiral DCB and standard PTA in 70 patients with SFA ISR [31]. The DCB group had significant reduction in percentage diameter stenosis at 6 to 8 months (44 ± 33% vs 65 ± 33%, p=0.01) and in CD-TLR at 24 months (19% vs 50%, p=0.007), compared to the PTA group. In the PACUBA (Paclitaxel Balloon Versus Standard Balloon in In-Stent Restenoses of the Superficial Femoral Artery) trial of angioplasty using FREEWAY DCB (Eurocor Tech GmbH, Bonn, Germany) versus PTA in 74 patients with femoropopliteal ISR [32], DCB angioplasty was associated with higher rates of primary patency (40.7% vs 13.4%, p=0.02) and freedom from CD-TLR (49.0% vs 22.1%, p=0.11) at 12 months, compared to PTA. A meta-analysis of patient-level data from these 3 RCTs (FAIR [30], ISAR-PEBIS [31], and PACUBA [32]) has recently been published [33]. Patients treated with DCB angioplasty exhibited a lower risk for CD-TLR (hazard ratio [HR] 0.25, 95% confidence interval [CI] 0.14–0.46, p<0.001) and recurrent ISR (HR 0.19, 95% CI 0.10–0.35, p<0.001) at 12-month follow-up. While this data suggests the superior efficacy of DCB over PTA for the treatment of femoropopliteal ISR
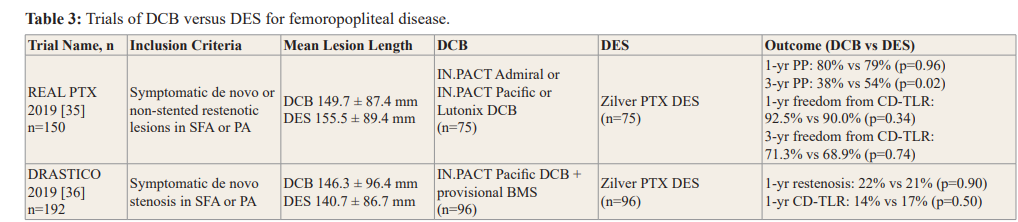
BMS: Bare-Metal Stent; CD-TLR: Clinically Driven Target Lesion Revascularization; DCB: Drug-Coated Balloon; DES: Drug-Eluting Stent; PA: Popliteal Artery; PP: Primary Patency.up to 1 year, there is still a need for further investigation into the long-term durability of DCB angioplasty in this setting.
DCB versus DES
Recent RCTs of DCB versus DES are summarised in Table 3. In a retrospective study of DCB and DES outcomes in 228 patients, restenosis and clinical outcomes were not significantly different at 1 year. Binary restenosis rates were 23.9% and 30.4% and CD- TLR rates were 15.6% and 19.0% in the DCB and DES groups, respectively [34]. The REAL PTX (Randomised Evaluation of the Zilver PTX Stent vs Paclitaxel-Eluting Balloons for Treatment of Symptomatic Peripheral Artery Disease of the Femoropopliteal Artery) study randomised 150 patients with femoropopliteal disease to either DCB with bailout stenting or DES. Patency rates were similar between DCB and DES (80% vs 79%, p=0.96) at 1 year but reduced to 38% and 54% at 3 years (p=0.02). Freedom from CD-TLR was high in both groups at 1 year (DCB 92.5% vs DES 90.0%, p=0.34) but decreased in both groups at 3 years (DCB 71.3% vs DES 68.9%, p=0.74) [35]. The DRASTICO (Drug-eluting balloon versus drug-eluting stent for Complex Femoropopliteal Arterial Lesions) study compared the use of DCB and DES in 192 patients randomised to paclitaxel DCB with bailout nitinol BMS or to paclitaxel DES. There was no significant difference between the two groups in target-lesion binary restenosis at 1 year (DCB 22% vs DES 21%) [36].
Discussion
The advent of drug-based strategies utilising DES and DCB has significantly reduced the restenosis and CD-TLR in patients with femoropopliteal disease. However, most of the evidence has been derived from relatively simple lesions and there is a paucity of data regarding complex, long or calcified disease. Even though stenting improved the outcome in intermediate- to long-length lesions compared to PTA, the risk of late complications such as ISR or stent fracture remains. DCB are associated with improved vessel patency and reduced stent use, but they frequently entail bailout stenting, particularly in complex lesions such as heavy calcification, chronic and long chronic total occlusions (CTO).
DCB in clinical practice
Currently, most DCB are coated with paclitaxel at a concentration between 2 and 3.5 μg/mm2 [17,18,26]. Paclitaxel is often used for DCB coating due to its lipophilic properties and resistance to oxidation. Three DCB are in clinical use for femoropopliteal disease: Lutonix (Bard Lutonix, New Hope, Minnesota, USA), IN.PACT (Medtronic Vascular, Santa Rosa, California, USA), and Stellarex (Royal Philips, Amsterdam, The Netherlands). These DCB use polysorbate and sorbitol, urea, and polyethylene glycol as excipients, respectively. These excipients are coated onto the balloon with paclitaxel to control the release of the drug into the arterial wall [37].
There are a number of technical challenges in the development of DCB including effective transfer of drug to the vessel wall, minimising the loss of drug during the catheter advancement and providing a long-term anti-restenosis effect. Clinical trials have revealed that the use of DCB was associated with superior patency rates to PTA at 1 year [5,17]. However, patency rates decreased significantly beyond the first year, suggesting a late catch-up phenomenon following DCB treatment [26]. Similarly, in the REAL PTX trial [35], although the patency and CD-TLR rates were not different between DCB and DES at 1 year, there was a trend in favour of DES at long-term follow-up with a higher 3-year patency rate with DES than DCB (56.7% vs 42.4%, p=0.17) [35]. Furthermore, there has been concern over the reduced efficacy of DCB in long, complex lesions including heavy calcification [38]. The usage of adjunctive technologies such as atherectomy to debulk the plaque prior to DCB has been suggested to improve results [39]. Large-scale studies with long-term follow-up are warranted to evaluate the efficacy and safety of DCB in these lesions, which often require bailout stenting.
In deciding between DCB and DES for the treatment of femoropopliteal lesions, current evidence suggests stratified strategies according to the lesion complexity; with DCB for short- or intermediate-length, non-CTO lesions and DES for long, heavily calcified or CTO lesions. Consensus statements have been published to help guide these decisions [40]. However, a great deal of variation in practice remains an issue, which warrants clarification with further confirmatory RCTs. Until then a reasonable approach could be to observe the vessel response after pre-dilatation with PTA with a view to using DCB in lesions without dissection or residual stenosis and to using DES in those with suboptimal results or in long, calcified lesions.
Concern over paclitaxel-coated devices
Recently, the safety of paclitaxel-based therapies in patients with PAD has been called into question. A systematic review and study- level meta-analysis suggested an increased risk for late mortality in patients treated with paclitaxel DCB and DES [41]. The study reviewed 28 RCTs (n=4,663) of paclitaxel DCB and DES in femoropopliteal arteries. There was no difference in mortality at 1 year. However, there was a higher risk of all-cause mortality at 2 years compared with controls (7.2% vs 3.8%, risk ratio [RR] 1.68, 95% CI 1.15-2.47). All-cause mortality at 5 years was also higher with the paclitaxel-coated devices (14.7% vs 8.1%, RR 1.93, 95% CI 1.27-2.93). A subsequent meta-analysis of individual patient- level data from 8 RCTs of US Food and Drug Administration (FDA)–approved paclitaxel-coated balloons (IN.PACT Admiral, Lutonix, Stellarex) and stents (Zilver PTX) observed a 4.6% increase in absolute risk of death associated with the use of paclitaxel-coated devices compared with PTA at a median 4-year follow-up [42]. With the recovery of lost-to-follow-up data, the mortality risk was slightly attenuated but still significantly higher for paclitaxel-coated devices compared with PTA (HR 1.27, 95% CI 1.03-1.58). The US FDA has recommended that paclitaxel- coated devices should be reserved for patients at the highest risk of restenosis and alternative treatment options should be considered until the safety of these devices can be verified [43]. The potential increased mortality, however, should be interpreted with caution due to the substantial limitations of these meta-analyses such as pooling of studies of different paclitaxel-coated devices, missing study data, lack of dose-response relationships, and no known mechanism for the increased mortality [44].
Recent large randomised and observational studies [45-48], and long-term follow-up data from the RCTs [49-51] have refuted the increased mortality signal observed in patients treated with paclitaxel-coated devices. In the SWEDEPAD (Swedish Drug Elution Trial in Peripheral Arterial Disease) RCT (n=2,289), all-because mortality was similar between the paclitaxel-coated and uncoated device groups at a mean follow-up of 2.5 years (drug-coated device: 25.5% vs uncoated device: 24.6%) [45]. In a retrospective analysis of the German BARMER insurance claims (n=37,914), the use of paclitaxel-coated balloons and stents were associated with improved survival at 5 years compared with uncoated devices (HR 0.83, 95% CI 0.77-0.90 in patients with chronic limb threatening ischaemia and HR 0.88, 95% CI 0.80- 0.98 in patients with intermittent claudication) [46]. Another study from the same database (n=64,771) showed no evidence for increased mortality associated with paclitaxel-coated devices for over 11 years [47]. In an analysis from the Society for Vascular Quality Initiative Registry (n=8,376), mortality was overall similar between paclitaxel-coated balloon and PTA cohorts (9.6% vs 12.6%, p=0.14) and between paclitaxel-coated stent and BMS cohorts (8.8% vs 9.8%, p=0.75) at a median follow-up of 12.6 months and 13.0 months, respectively [48]. Long-term follow-up results from RCTs have also not shown an increased mortality signal. In a patient-level meta-analysis of IN.PACT trials comprised of 2 RCTs (IN.PACT SFA and IN.PACT SFA Japan) and 2 prospective single-arm studies (IN.PACT SFA China, IN.PACT Global) (n=1,980), there was no significant difference in all-cause mortality between DCB and PTA through 5 years (15.12% DCB vs 11.15% PTA) [49]. An analysis of individual patient-level data from the LEVANT trials (n=1,343) consisting of 3 RCTs (LEVANT 1, LEVANT 2, and LEVANT Japan Clinical Trial and a single arm continued-access arm of LEVANT 2) demonstrated no increase in mortality with the use of DCB compared to PTA at 5 years [50]. Analyses of the Zilver PTX patient-level data (n=479) showed no difference in all-cause mortality between DES and PTA/BMS cohorts through 5 years (19.1% vs 17.1%, p = 0.60) [51].
Future Directions
There has been great interest in ‘limus’ compounds as a potential substitute for paclitaxel in the DCB and DES arena [52]. These have broader therapeutic window [53] and may have an advantage over paclitaxel in safety outcomes. The SELUTION DCB is the first-in-human study (NCT02941224) currently underway of a sirolimus-coated balloon [SELUTION™ (MA Med Alliance SA, Mont-sur-Rolle, Switzerland)] in femoropopliteal disease. Recently, there have been efforts to improve stent designs by applying interwoven nitinol mesh, helical flow, and high flexibility in order to enhance long-term patency and reduce the risk of stent fracture [54-56]. Their utility as a DES platform or in adjunct to DCB should be validated in future studies. Furthermore, bioresorbable vascular scaffolds hold promise as another initiative within the leave-nothing-behind strategy, even though there is room for improvement regarding the maintenance of mechanical integrity and optimal resorption [57].
Conclusions
In patients with atherosclerotic femoropopliteal disease, DCB and DES provide clear benefits over PTA and recent studies indicate that both of these drug-based approaches may be effective with comparable outcomes up to 1 year. Even though a leave-nothing- behind strategy with the use of DCB may appear desirable, the need for bailout stenting will increase as lesions become more complex. Further RCTs will be needed to assess long-term outcomes of DCB in comparison with DES. Until then, lesion characteristics alongside vessel response to PTA should help guide the decision on the optimal drug-based endovascular treatment strategy for this patient population.
References
- Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013; 382: 1329-1340.
- Bishu K, Armstrong EJ. Supera self-expanding stents for endovascular treatment of femoropopliteal disease: a review of the clinical evidence. Vasc Health Risk Manag. 2015; 11: 387-395.
- Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018; 39: 763-816.
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017; 69: e71-e126.
- Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015; 131: 495-502.
- Krankenberg H, Schluter M, Steinkamp HJ, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation. 2007; 116: 285-292.
- Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010; 3: 267-276.
- Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006; 354: 1879-1888.
- Tosaka A, Soga Y, Iida O, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012; 59: 16-23.
- Sarode K, Spelber DA, Bhatt DL, et al. Drug delivering technology for endovascular management of infrainguinal peripheral artery disease. JACC Cardiovasc Interv. 2014; 7: 827-839.
- Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011; 4: 495-504.
- Dake MD, Ansel GM, Jaff MR, et al. Durable Clinical Effectiveness With Paclitaxel-Eluting Stents in the Femoropopliteal Artery: 5-Year Results of the Zilver PTX Randomized Trial. Circulation. 2016; 133: 1472-1483.
- Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008; 358: 689-699.
- Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008; 118: 1358-1365.
- Werk M, Albrecht T, Meyer DR, et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012; 5: 831-840.
- Liistro F, Grotti S, Porto I, et al. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC Cardiovasc Interv. 2013; 6: 1295-1302.
- Rosenfield K, Jaff MR, White CJ, et al. Trial of a Paclitaxel- Coated Balloon for Femoropopliteal Artery Disease. N Engl J Med. 2015; 373: 145-153.
- Brodmann M, Werner M, Meyer DR, et al. Sustainable Antirestenosis Effect With a Low-Dose Drug-Coated Balloon: The ILLUMENATE European Randomized Clinical Trial 2-Year Results. JACC Cardiovasc Interv. 2018; 11: 2357- 2364.
- Ansari F, Pack LK, Brooks SS, et al. Design considerations for studies of the biomechanical environment of the femoropopliteal arteries. J Vasc Surg. 2013; 58: 804-813.
- Virmani R, Liistro F, Stankovic G, et al. Mechanism of late in-stent restenosis after implantation of a paclitaxel derivate- eluting polymer stent system in humans. Circulation. 2002; 106: 2649-2651.
- Muller-Hulsbeck S, Keirse K, Zeller T, et al. Twelve-Month Results From the MAJESTIC Trial of the Eluvia Paclitaxel- Eluting Stent for Treatment of Obstructive Femoropopliteal Disease. J Endovasc Ther. 2016; 23: 701-707.
- Muller-Hulsbeck S, Keirse K, Zeller T, et al. Long-Term Results from the MAJESTIC Trial of the Eluvia Paclitaxel- Eluting Stent for Femoropopliteal Treatment: 3-Year Follow- up. Cardiovasc Intervent Radiol. 2017; 40: 1832-1838.
- Gray WA, Keirse K, Soga Y, et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet. 2018; 392: 1541-1551.
- Duda SH, Bosiers M, Lammer J, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006; 13: 701-710.
- Goueffic Y, Sauguet A, Desgranges P, et al. A Polymer-Free Paclitaxel-Eluting Stent Versus a Bare-Metal Stent for De Novo Femoropopliteal Lesions: The BATTLE Trial. JACC Cardiovasc Interv. 2020; 13: 447-457.
- Schneider PA, Laird JR, Tepe G, et al. Treatment Effect of Drug-Coated Balloons Is Durable to 3 Years in the Femoropopliteal Arteries: Long-Term Results of the IN.PACT SFA Randomized Trial. Circ Cardiovasc Interv. 2018; 11: e005891.
- Scheinert D, Schulte KL, Zeller T, et al. Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve-month results from the BIOLUX P-I randomized trial. J Endovasc Ther. 2015; 22: 14-21.
- Steiner S, Willfort-Ehringer A, Sievert H, et al. 12-Month Results From the First-in-Human Randomized Study of the Ranger Paclitaxel-Coated Balloon for Femoropopliteal Treatment. JACC Cardiovasc Interv. 2018; 11: 934-941.
- Teichgraber U, Lehmann T, Aschenbach R, et al. Drug-coated Balloon Angioplasty of Femoropopliteal Lesions Maintained Superior Efficacy over Conventional Balloon: 2-year Results of the Randomized EffPac Trial. Radiology. 2020; 295: 478- 487.
- Krankenberg H, Tubler T, Ingwersen M, et al. Drug-Coated Balloon Versus Standard Balloon for Superficial Femoral Artery In-Stent Restenosis: The Randomized Femoral Artery In-Stent Restenosis (FAIR) Trial. Circulation. 2015; 132: 2230-2236.
- Ott I, Cassese S, Groha P, et al. ISAR-PEBIS (Paclitaxel- Eluting Balloon Versus Conventional Balloon Angioplasty for In-Stent Restenosis of Superficial Femoral Artery): A Randomized Trial. J Am Heart Assoc. 2017; 6: e006321.
- Kinstner CM, Lammer J, Willfort-Ehringer A, et al. Paclitaxel- Eluting Balloon Versus Standard Balloon Angioplasty in In- Stent Restenosis of the Superficial Femoral and Proximal Popliteal Artery: 1-Year Results of the PACUBA Trial. JACC Cardiovasc Interv. 2016; 9: 1386-1392.
- Cassese S, Wolf F, Ingwersen M, et al. Drug-Coated Balloon Angioplasty for Femoropopliteal In-Stent Restenosis. Circ Cardiovasc Interv. 2018; 11: e007055.
- Zeller T, Rastan A, Macharzina R, et al. Drug-coated balloons vs. drug-eluting stents for treatment of long femoropopliteal lesions. J Endovasc Ther. 2014; 21: 359-368.
- Bausback Y, Wittig T, Schmidt A, et al. Drug-Eluting Stent Versus Drug-Coated Balloon Revascularization in Patients With Femoropopliteal Arterial Disease. J Am Coll Cardiol. 2019; 73: 667-679.
- Liistro F, Angioli P, Porto I, et al. Drug-Eluting Balloon Versus Drug-Eluting Stent for Complex Femoropopliteal Arterial Lesions: The DRASTICO Study. J Am Coll Cardiol. 2019; 74: 205-215.
- Li J, Karim A, Shishehbor MH. The use of drug-coated balloons in the treatment of femoropopliteal and infrapopliteal disease. J Cardiovasc Surg (Torino). 2018; 59: 512-525.
- Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014; 37: 898-907.
- Zeller T, Langhoff R, Rocha-Singh KJ, et al. Directional Atherectomy Followed by a Paclitaxel-Coated Balloon to Inhibit Restenosis and Maintain Vessel Patency: Twelve- Month Results of the DEFINITIVE AR Study. Circ Cardiovasc Interv. 2017; 10: e004848.
- Feldman DN, Armstrong EJ, Aronow HD, et al. SCAI consensus guidelines for device selection in femoral-popliteal arterial interventions. Catheter Cardiovasc Interv. 2018; 92: 124-140.
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2018; 7: e011245.
- Rocha-Singh KJ, Duval S, Jaff MR, et al. Mortality and Paclitaxel-Coated Devices: An Individual Patient Data Meta- Analysis. Circulation. 2020; 141: 1859-1869.
- https://www.fda.gov/medical-devices/letters-health-care-providers/update-treatment-peripheral-arterial-disease-paclitaxel-coated-balloons-and-paclitaxel-eluting
- Beckman JA, White CJ. Paclitaxel-Coated Balloons and Eluting Stents: Is There a Mortality Risk in Patients With Peripheral Artery Disease?. Circulation. 2019; 140: 1342-1351.
- Nordanstig J, James S, Andersson M, et al. Mortality with Paclitaxel-Coated Devices in Peripheral Artery Disease. N Engl J Med. 2020; 383: 2538-2546.
- Behrendt CA, Sedrakyan A, Peters F, et al. Editor's Choice - Long Term Survival after Femoropopliteal Artery Revascularisation with Paclitaxel Coated Devices: A Propensity Score Matched Cohort Analysis. Eur J Vasc Endovasc Surg. 2020; 59: 587-596.
- Freisinger E, Koeppe J, Gerss J, et al. Mortality after use of paclitaxel-based devices in peripheral arteries: a real-world safety analysis. Eur Heart J. 2020; 41: 3732-3739.
- Bertges DJ, Sedrakyan A, Sun T, et al. Mortality After Paclitaxel Coated Balloon Angioplasty and Stenting of Superficial Femoral and Popliteal Artery in the Vascular Quality Initiative. Circ Cardiovasc Interv. 2020; 13: e008528.
- Schneider PA, Laird JR, Doros G, et al. Mortality Not Correlated With Paclitaxel Exposure: An Independent Patient- Level Meta-Analysis of a Drug-Coated Balloon. J Am Coll Cardiol. 2019; 73: 2550-2563.
- Ouriel K, Adelman MA, Rosenfield K, et al. Safety of Paclitaxel-Coated Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease. JACC Cardiovasc Interv. 2019; 12: 2515-2524.
- Dake MD, Ansel GM, Bosiers M, et al. Paclitaxel-Coated Zilver PTX Drug-Eluting Stent Treatment Does Not Result in Increased Long-Term All-Cause Mortality Compared to Uncoated Devices. Cardiovasc Intervent Radiol. 2020; 43: 8-19.
- Lemos PA, Farooq V, Takimura CK, et al. Emerging technologies: polymer-free phospholipid encapsulated sirolimus nanocarriers for the controlled release of drug from a stent-plus-balloon or a stand-alone balloon catheter. EuroIntervention. 2013; 9: 148-156.
- Bozsak F, Gonzalez-Rodriguez D, Sternberger Z, et al. Optimization of Drug Delivery by Drug-Eluting Stents. PLoS One. 2015; 10: e0130182.
- Garcia LA, Rosenfield KR, Metzger CD, et al. SUPERB final 3-year outcomes using interwoven nitinol biomimetic supera stent. Catheter Cardiovasc Interv. 2017; 89: 1259-1267.
- Zeller T, Gaines PA, Ansel GM, et al. Helical Centerline Stent Improves Patency: Two-Year Results From the Randomized Mimics Trial. Circ Cardiovasc Interv. 2016; 9: e002930.
- Laird JR, Zeller T, Loewe C, et al. Novel Nitinol Stent for Lesions up to 24 cm in the Superficial Femoral and Proximal Popliteal Arteries: 24-Month Results From the TIGRIS Randomized Trial. J Endovasc Ther. 2018; 25: 68-78.
- Lammer J, Bosiers M, Deloose K, et al. Bioresorbable Everolimus-Eluting Vascular Scaffold for Patients With Peripheral Artery Disease (ESPRIT I): 2-Year Clinical and Imaging Results. JACC Cardiovasc Interv. 2016; 9: 1178-1187.