Effect of Classic or Delayed Ischemic Conditioning on Left Ventricular Pressure-Volume Relations in Dogs
Author'(s): Kingma J.G. Jr.*, Simard D and Rouleau J.R.
Department of Medicine, Faculty of Medicine, Laval University Québec, QC G1K 7P4, Canada.
*Correspondence:
JG Kingma Jr, Ph.D., Centre de Recherche, Institut universitaire de, Cardiologie et de Pneumologie de Québec, Université Laval, 2725, Chemin Sainte-Foy, Sainte-Foy (Qc), G1V 4G5, Canada,Tel: (418) 656-8711, ORCID ID: 0000-0002-3917-4417.
Received: 28 Apr 2022; Accepted: 24 May 2022; Published: 30 May 2022
Citation: Kingma JG, Simard D, Rouleau JR. Effect of Classic or Delayed Ischemic Conditioning on Left Ventricular Pressure-Volume Relations in Dogs. Cardiol Vasc Res. 2022; 6(3): 1-8.
Abstract
Objective: Non-pharmacologic interventions such as ischemic conditioning (IC) markedly reduce infarct size but it remains unclear whether this protection translates to a significant attenuation of ischemia-mediated LV contractile dysfunction. In this study we evaluated pressure-volume (P-V) data obtained by the load-insensitive conductance catheter method, to examine LV pressure-volume (LVP-V), diastolic function and ventricular-arterial coupling in anesthetized dogs exposed to ischemic conditioning (IC) or delayed IC (dIC; 48h prior to ischemia). The objective was to determine if IC, or dIC pre-treatment could influence post-ischemic recovery of LV contractile function.
Methods: Three groups were studied – nIC, IC and dIC; all dogs underwent 90-min acute coronary occlusion (CO) followed by 180-min reperfusion (REP). IC consisted of 4 cycles of 5-min CO and 5-min REP of the left main coronary artery. LV P-V relations were constructed under steady-state conditions (by transient occlusion of the inferior vena cava) prior to IC treatment and at the end of the experiment; P-V loop data was also acquired at different points during the experiment to evaluate changes in end-systolic and end-diastolic parameters.
Results: During CO dP/dtmax and dP/dtmin (indicator of rate of LV relaxation) decreased significantly compared to baseline in the IC group; these variables were unchanged in the dIC group. LVEF decreased during CO and REP in the nIC and IC groups but was stable in the dIC group. Tau was increased only in the IC group during CO and REP. ESV and EDV increased significantly in all groups during CO and REP; none of these negative effects was resorbed by the end of the experimental period.
Conclusions: Diminished LV contractile function caused by CO did not improve with IC, or dIC pre-treatment, despite significant reduction in infarct size.
Keywords
Introduction
Myocardial infarction causes left ventricular remodeling that over time produces progressive heart failure, which leads to persistent left ventricle (LV) contractile dysfunction. Mechanisms involved include changes in LV chamber dimensions and geometry as well as hemodynamic modifications that decrease cardiac performance [1]. Pharmacologic interventions designed to improve post-ischemic cardiac function are currently being investigated and recommendations from international medical associations advocate their use in patients due to proven benefits for reducing mortality [2,3]. Non-pharmacologic interventions such as ischemic conditioning (IC) can also markedly reduce infarct size in humans and almost all animal species studied [4- 6]. Whether significant restoration of LV contractile function can be achieved by IC remains uncertain based on studies performed in globally ischemic isolated rabbit hearts [7] and in in situ canine and porcine hearts [8,9]. In the present study, we used LV pressure-volume (LVP-V) measurements to evaluate changes in systolic and diastolic parameters of LV contractile performance. For these studies in anesthetised dogs were subjected to IC (classic or delayed - dIC) prior to a prolonged coronary occlusion (CO) followed by reperfusion (REP). Our findings provide additional information on the efficacy of non-pharmacologic interventions on post-ischemic LV contractile function.
Materials and Methods
Dogs were acquired through the Division of Laboratory Animal Services at Laval University; they were housed in individual cages under conditions of constant temperature and humidity and kept on a strict 12:12 h dark light cycle. Dogs had free access to food and water. This study was approved (#2007-001-2) by the institutional animal welfare committee at Laval University (A5012-01) and was carried out in compliance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (publication 85-23; revised 1996). Experiments were carried out, and results reported as described in the ARRIVE guidelines [10].
Surgical preparation
Anesthesia protocols are described in earlier studies from our laboratory [11-14]. Briefly, dogs (both male and female; 20-25 Kg) were intubated and anesthesia was maintained with isoflurane (1-2%) and oxygen-enriched room air. Fentanyl (0.005 mg/Kg IV bolus followed by constant infusion at 0.005 mg/Kg/h) was administered for analgesia. Normothermia was maintained with a water-jacketed Micro-Temp heating blanket (Zimmer, Dover, OH, USA); saline was given (250 mL/h IV) to replace fluid loss.
Dogs were placed in the supine position and vascular introducer sheaths (8Fr, Terumo Medical Corp. USA) were placed in the left and right femoral arteries; a triple-lumen central venous catheter (7Fr, Arrow-HowesTM, Arrow Intl. Inc., Reading, PA, USA) was positioned in the right femoral vein. A left lateral thoracotomy was performed through the fifth intercostal space; the heart was exposed and suspended in a pericardial cradle. A section of the left anterior descending artery branch (distal to the first diagonal branch) was dissected to allow placement of a vascular clamp for regional coronary artery occlusion. Umbilical tape was placed around the inferior vena cava (IVC) cranial to the diaphragm. This allowed for brief IVC occlusion later in the experiment for construction of LVP-V relations. A catheter (7Fr) was advanced into the main pulmonary artery (for determination of parallel conductance using hypertonic saline) [15]. A solid-state pressure transducer (5Fr, MPC500, Millar Instruments Inc., Houston, TX, USA) was placed in the LV cavity via an apical approach. A 12-electrode conductance catheter (7Fr, Leycom, Oegstergeest, The Netherlands) was advanced (via femoral artery) to the LV apex along the longitudinal axis of the ventricle as previously described [16]. Steady state LVP-V loops were recorded during normal sinus rhythm and apnea; blood resistivity was measured prior to acquisition of conductance catheter data using a resistivity cuvette (Leycom, Oegstergeest, The Netherlands). Bolus heparin sodium (500 IU, IV) was given, followed by hourly (100 IU, IV) administrations to prevent undue blood clotting after all catheters were positioned. After completion of surgical procedures, a 30-min stabilization period was allowed before initiation of the experimental protocol. For the dIC study, dogs were pre-medicated with diazepam (1 mg/ Kg IV) and fentanyl (20 µg/Kg IV) and anesthetized with 1.5- 2.0% isoflurane. IC was performed by coronary catheterization
[17]; briefly, the right femoral artery was cannulated with a 7F sheath for percutaneous transluminal coronary angioplasty and 1000 IU heparin administered IV. Under angiographic guidance, a 6F guiding catheter was inserted and advanced to the coronary ostium. Left coronary angiography (oblique view) was performed to delineate the left main coronary artery and its branches; a floppy guide wire (0.0014 inch) was advanced into the left main circumflex artery and an inflatable balloon catheter was positioned just distal to the first marginal branch (defined by angiography). Ischemic conditioning (4 cycles of 5-min CO and 5-min REP) was done by balloon inflation (3 bars)/deflation; 1-min after onset of CO or REP contrast medium was injected and an angiogram obtained to verify absence/restoration of blood flow within the infarct-related artery. After completion of IC, catheters were removed and tissue and skin incisions sutured; dogs were weaned from the respirator and extubated after restoration of a regular breathing pattern. Each dog was administered antibiotic (cefazoline sodium, 5.5 mg/Kg, im), analgesic (buprenorphine, 0.05 mg/Kg, im) and heparin (500 IU, sc) before returning to the recovery room. After 48h recovery, dogs underwent the above-described surgical procedure for ischemia- reperfusion.
Experimental Protocol
Dogs (n=24) were randomly assigned to control (nIC), ischemic conditioning (IC), or delayed ischemic conditioning (dIC) groups as shown in Figure 1. In the nIC group, a 40-min wait period was instituted to allow comparisons between treatments. In the IC groups, dogs were subject to 4 cycles of 5-min CO and 5-min coronary REP prior to prolonged CO [6].
For all dogs, LVP-V loops were recorded under steady-state conditions (during apnea) prior to CO and at the end of the experimental period (cf. Figure 1); LVP-V loops were obtained by transient occlusion of the IVC [18,19]. Dogs underwent 90- min regional CO followed by 180-min REP; xylocaine was administered (10 mg IV bolus; Astra Pharma, Inc., Mississauga, ONT, CAN) after 30-min of CO and just prior to REP to limit ischemia- or reperfusion-induced arrhythmias. Hearts that fibrillated were cardioverted (DC shock ≤50 Joules) with a cardiac defibrillator (General Electric); if defibrillation was not successful after two attempts, the animal was euthanized and not entered into the data analysis. The Millar solid-state pressure transducer was cross calibrated with both systolic aortic and diastolic left atrial pressure; the conductance catheter was connected to a Sigma 5DF signal conditioning and processing unit (Leycom, Oegstergeest, The Netherlands). All data were recorded continuously and stored on computer hard drive for later analysis.
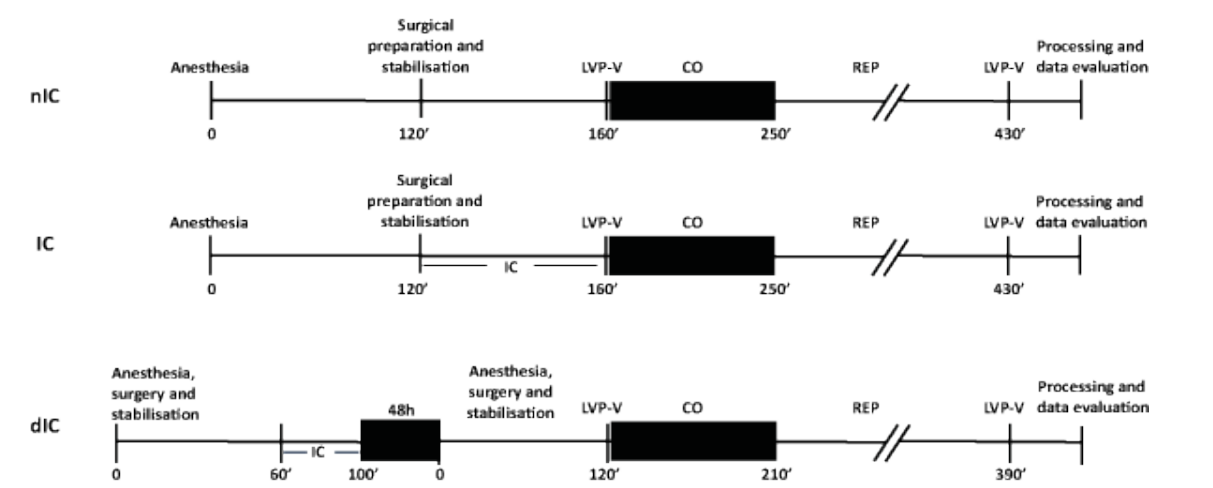
Figure 1: Timeline of the experimental protocol.
Statistical analysis
Data are expressed as means ± 1SD. Data normality was verified by the Shapiro-Wilk test (after Cholesky factorization); a linear mixed effects model was used to identify changes in cardiac variables. A repeated-measure ANOVA (linear mixed model) allowed determination of statistical differences; selection of a covariance structure was based on the Akaike information criterion. We compared data with a Tukey’s test when interactions were not significant. A p value <0.05 was considered statistically significant for all analyses. Statistical analyses were performed using the statistical packages R v3.0.2 (R Foundation for Statistical Computing, Vienna, and Austria.) and SAS v9.4 (SAS Institute Inc., Cary, NC, U.S.A.).
Results
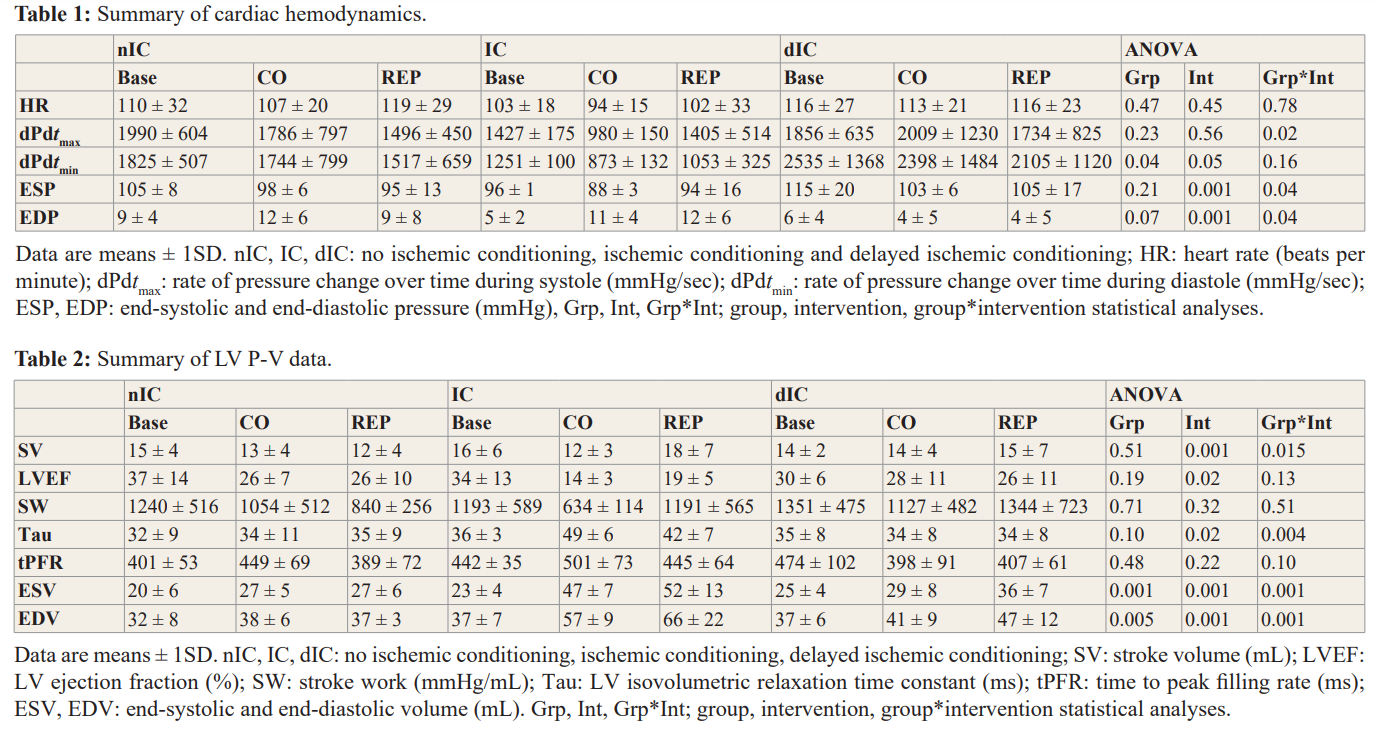
Twenty-four dogs (n= 8 per group) completed the experimental protocol. The incidence of ventricular arrhythmias during ischemia/ reperfusion (2/8 nIC; 1/8 IC; 1/8 dIC) but is not markedly different between groups. Infarct size (necrosis as percent of risk area) was smaller in IC and dIC groups (32 ± 10 nIC; 17 ± 6 IC; 17 ± 6 dIC; p=0.01 vs nIC) [11]. A summary of changes in cardiac dynamics is presented in Table 1. Heart rate (HR) was stable during CO and REP in each experimental group. During CO, dP/dtmax decreased significantly in the IC (versus baseline) group; however, results were not changed in the nIC and dIC groups, respectively. A similar pattern was observed for dP/dtmin for the IC group. LV end-systolic pressure (ESP) decreased markedly during CO in all groups. LV end-diastolic (EDP) pressure increased in the IC group during CO and REP; however, similar changes did not occur in the nIC or dIC groups.
Changes in stroke volume (SV) varied significantly in IC dogs during CO and REP (Table 2). LV ejection fraction (LVEF) decreased significantly during CO and REP in nIC and IC groups indicating impaired LV systolic performance (also supported by reductions in dP/dtmax); however, LVEF remained stable in the dIC group during the experiment. Stroke work (SW) decreased during CO but did not achieve a level of statistical significance; however, at the end of the experimental protocol some recovery to near baseline values was observed in IC and dIC groups. Tau (i.e. time constant of LV relaxation) was significantly higher during CO and REP in the IC group with no change in either nIC or dIC dogs; tPFR (time to peak filling rate) was unchanged for all groups. LV end-systolic volume (ESV) increased significantly during CO and REP in all groups; we observed a similar pattern for LV end- diastolic volume (EDV).
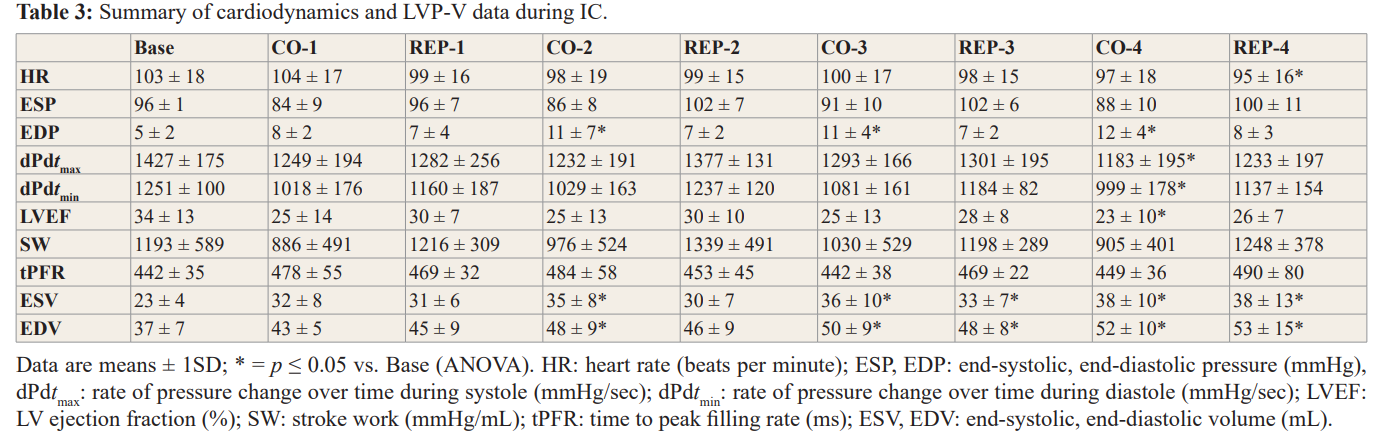
A summary of changes in cardiodynamics and LVP-V data afforded by IC treatment is reported in Table 3. HR was markedly lower than baseline values at the end of REP-4; ESP was not changed (compared to baseline) but EDP increased significantly during each CO cycle and subsequently returned to normal values during REP. dPdtmax and dPdtmin decreased during CO-4 but were otherwise not markedly affected. LVEF (global indicator of LV contractile performance) also decreased significantly during CO-4. No change in SW or tPFR were observed during the IC protocol. ESV and EDV increased significantly beginning with CO-2 and remained higher than baseline values from CO-3 to the end of the IC protocol. These findings suggest a gradual reduction of LV function caused by IC; however, the underlying cause has not been determined since it is generally acknowledged that myocyte injury (i.e. necrosis) produced by IC treatment is limited.
LV elastance at end-systole (Ees; mm Hg/mL) of the LVP-V relation did not change but the LV volume intercept (V0; mL) was shifted to the right in all groups after ischemia-reperfusion principally due to higher ESV and EDV as shown in Figure 2. No improvement in this relation (i.e. leftward shift) was observed for the IC or dIC groups. Rightward shift of LVP-V loops at different time periods during the experiment, with attendant reduction of LV contractility caused by CO, are illustrated in Figure 3; LV contractile function in dogs pretreated with either IC or dIC was not restored to baseline levels.
Discussion
Pharmacologic or non-pharmacologic interventions that afford protection against ischemia-induced myocardial injury should hypothetically allow for more rapid restoration of post-ischemic cardiac contractile function. In this study, in dogs subject to prolonged CO, IC or dIC did not markedly improve post-ischemic LV contractile function even though infarct size is significantly reduced.
Normal LV function depends on intricate interactions between contractility, heart rate, pre- and after-load [20]. Since blood pressure is highly regulated minor deviations from the norm can result in serious complications. Both systolic and diastolic ventricular properties depend on muscle mass, LV chamber architecture and geometry [21]. Abnormal LVEF demonstrates incapacity of the cardiovascular system to modulate contractile and loading conditions that are essential for normal homeostasis. However, LVEF is an unreliable marker of LV intrinsic contractility as it is strongly influenced by LV loading conditions [22]. Consequently, LVEF is a ventriculo-arterial coupling index principally related to LV mechanical efficiency [23]. In the present study, ischemia causes marked reduction of LVEF that does not improve by IC pre-treatment; reperfusion did not restore LVEF to baseline, or near-baseline values. Interestingly, post-ischemic LV contractile dysfunction appears to be less affected in dogs exposed to dIC.
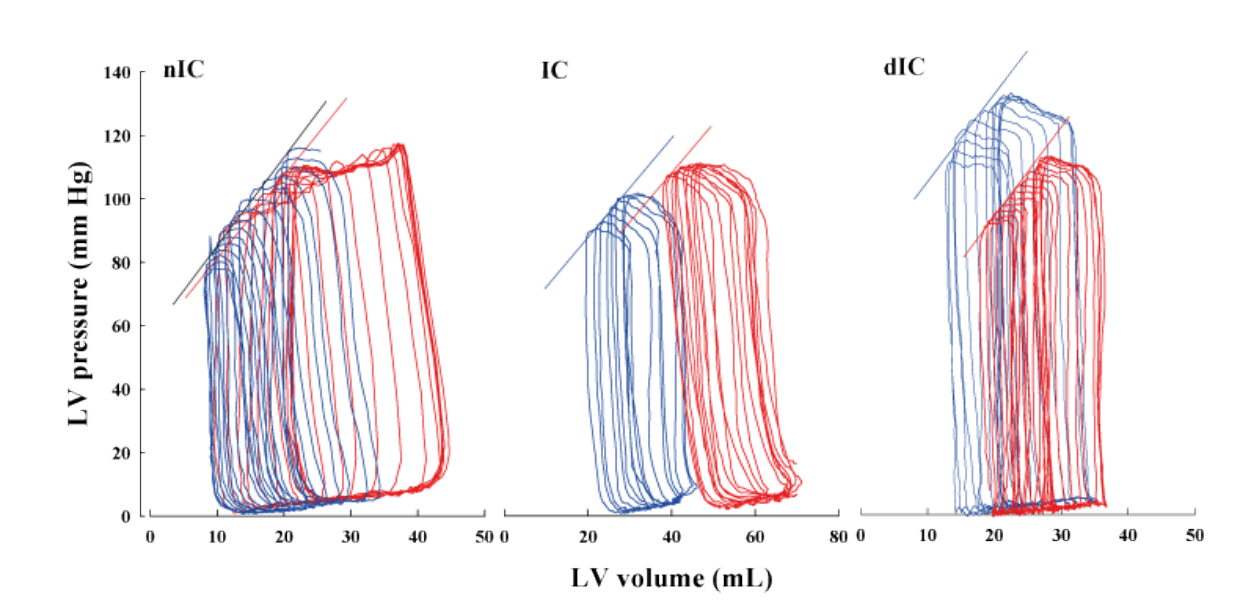
Figure 2: Representative steady-state LVP-V loops generated by transient IVC occlusion in nIC (left panel), IC (middle panel) and dIC (right panel) dogs at baseline (blue line) and after 180-min REP (red line); slopes of end-systolic LVP-V points are shown. In nIC dogs, Ees was 2.09 mm Hg/mL (r2 = 0.99) before and 2.64 mm Hg/mL (r2 = 0.96) post-ischemia (p=NS). In IC dogs, E was 1.73 mm Hg/mL (r2 = 0.98) before and 0.99 mm Hg/mL (r2 = 0.94) post-ischemia (p=NS). In dIC dogs, E was 3.80 mm Hg/mL (r2 = 0.95) before and 2.95 mm Hg/mL (r2 = 0.98) post-ischemia (p=NS). The LVP-V relation is shifted rightward after CO in all groups consistent with increased ESV and EDV.
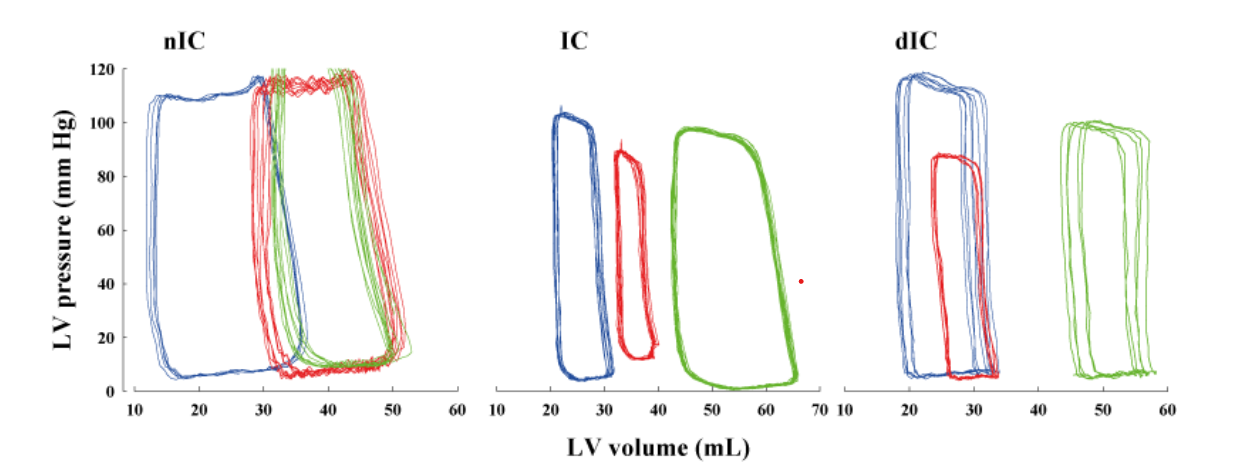
Figure 3: Representative LVP-V loops at baseline (blue line), 90-min CO (green line) and 180-min REP (red line) in nIC (left panel), IC (middle panel) and dIC (right panel) groups. During CO narrowing of LVP-V loops is due to a reduction in SW and shifts to the right are due to increased ventricular volume; IC or dIC did not significantly improve LV contractile status.
Post-ischemic myocardial contractile dysfunction, more commonly referred to as ‘myocardial stunning’ is a persistent mechanical dysfunction that occurs despite absence of irreversible myocyte damage. By definition, stunning denotes a fully reversible abnormality. Repetitive CO used in IC reported decreases LV contractile function (without irreversible cellular damage) beginning with the first ischemic insult [24]. Our findings indicate a restoration to near normal values of LV contractile function after IC and dIC with the exception of LVEF; the global derangement of LV function involves both systolic and diastolic function. The cumulative effects of repetitive ischemia on recovery of post-ischemic function are not thoroughly investigated. That being stated, IC does 1- afford robust improvement, 2- not change or 3- exacerbate LV contractile dysfunction post-ischemia [25-30].
Many of the positive studies used ultrasonic crystal micrometric methods (segment shortening, wall thickening, etc.) to measure regional myocardial function in small animal models. Various studies compared in situ LV contractile function using sonomicrometry or conductance catheter methods [31-33]; overall consensus is that different techniques for measurement of LV contractile function provide valid data and differences between methods are relatively insignificant. The conductance catheter method (i.e. gold standard for in vivo determination of systolic contractile performance in the intact heart) provides a comprehensive evaluation of LV contractile function [21,34]. This method enables continuous LVP-V loop analysis to describe intrinsic ventricular pump properties that are independent of preload, afterload [35-37] and within limits of heart rate, in the normal LV volume range [35,38-40]; however, corrections based on differences in infarct size may also be necessary [41,42]. End-systolic P-V relations provide information that includes end-systolic elastance and volume at zero pressure [43].
This study has some limitations; we used dogs with a health profile distinctly different from humans with heart disease and other comorbidities. Dogs are comparable to humans with respect to heart size and coronary physiology; existence of a large and diverse data bank using canine experimental models also favors comparisons between studies. Sample size, in the present study, is small (determined on basis of protection against ischemic injury) and the post-ischemic follow-up period much shorter that necessary to provide an adequate evaluation of potential recovery of LV function. Second, we used an open-chest preparation in anesthetised dogs; anesthetic agents are documented to protect against ischemic injury [44-46] and might potentially improve post-ischemic LV contractile function [47,48].
Conclusions
In the present study using the conductance catheter method we did not show marked improvement of global LV contractile function by either IC or dIC in dog hearts subject to a relatively prolonged period of CO. However, significant protection against myocardial necrosis occurs, as expected with IC and dIC. Whether pharmacologic or non-pharmacologic interventions can influence post-ischemic LV contractile function remains inconclusive; however, since ischemic durations of variable length cumulatively affect global LV contractile function further studies are encouraged.
Acknowledgements
The authors acknowledge Serge Simard for providing the statistical analyses of the experimental data used in this report. This study was supported by an operating grant from the Heart and Stroke Foundation of Québec.
References
- Angeli FS, Shapiro M, Amabile N, et al. Left ventricular remodeling after myocardial infarction: characterization of a swine model on beta-blocker therapy. Comp Med. 2009; 59: 272-279.
- Van de Werf F, Ardissino D, Betriu A, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2003; 24: 28-66.
- Antman EM, Hand M, Armstrong PW, et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008; 117: 296-329.
- Kingma JG. Conditioning strategies limit cellular injury?. World Journal of Cardiovascular Diseases. 2014; 4: 539-547.
- Kloner RA, Rezkalla SH. Preconditioning, postconditioning and their application to clinical cardiology. Cardiovasc Res. 2006; 70: 297-307.
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circ. 1986; 74: 1124-1136.
- Hendrikx M, Toshima Y, Mubagwa K, et al. Improved functional recovery after ischemic preconditioning in the globally ischemic rabbit heart is not mediated by adenosine A1 receptor activation. Basic Res Cardiol. 1993; 88: 576-593.
- Ovize M, Przyklenk K, Hale SL, et al. Preconditioning does not attenuate myocardial stunning. Circ. 1992; 85: 2247-2254.
- Kimura Y, Iyengar J, Subramanian R, et al. Preconditioning of the heart by repeated stunning: attenuation of postischemic dysfunction. Basic Res Cardiol. 1992; 87: 128-138.
- Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8: e1000412.
- Kingma JG. Inhibition of Na(+)/H(+) Exchanger With EMD 87580 does not Confer Greater Cardioprotection Beyond Preconditioning on Ischemia-Reperfusion Injury in Normal Dogs. J Cardiovasc Pharmacol Ther. 2018; 23: 254-269.
- Kingma JG, Jr Simard D, Rouleau JR. Nitric oxide bioavailability affects cardiovascular regulation dependent on cardiac nerve status. Auton Neurosci. 2015; 187: 70-75.
- Kingma JG, Jr Simard D, Voisine P, et al. Impact of chronic kidney disease on myocardial blood flow regulation in dogs. Nephron Exp Nephrol. 2014; 126: 175-182.
- Kingma JG, Simard D, Voisine P, et al. Influence of cardiac decentralization on cardioprotection. PLoS One. 2013; 8: e79190.
- Baan J, van der Velde E, de Bruin HG, et al. Continuous measurement of left ventricular volume in animls and humans by conductance catheter. Circ. 1984; 70: 812-823.
- Baan J, Van der Velde ET, Steendijk P. Ventricular pressure- volume relations in vivo. Eur Heart J. 1992; 13: 2-6.
- Jaberansari MT, Baxter GF, Muller CA, et al. Angiotensin- converting enzyme inhibition enhances a subthreshold stimulus to elicit delayed preconditioning in pig myocardium. J Am Coll Cardiol. 2001; 37: 1996-2001.
- Van der Linden LP, Van der Velde ET, Van Houwelingen HC, et al. Determinants of end-systolic pressure during different load alterations in the in situ left ventricle. Am J Physiol (Heart Circ Physiol). 1994; 267: H1895-H1906.
- Cingolani OH, Kass DA. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol. 2011; 301: h3198-h3206.
- Lankhaar JW, Rovekamp FA, Steendijk P, et al. Modeling the instantaneous pressure-volume relation of the left ventricle: a comparison of six models. Ann Biomed Eng. 2009; 37: 1710-1726.
- Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005; 289: H501-H512.
- Konstam MA, Abboud FM. Ejection Fraction: Misunderstood and Overrated (Changing the Paradigm in Categorizing Heart Failure). Circulation. 2017; 135: 717-719.
- Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res. 1985; 56: 586-595.
- Cohen MV, Downey JM. Myocardial stunning in dogs: preconditioning effect and influence of coronary collateral flow. Am Heart J. 1990; 120: 282-291.
- Cave AC, Apstein CS. Preconditioning-induced protection against post-ischemic contractile dysfunction: inhibitory effect of tissue washout. Basic Res Cardiol. 1996; 91: 16-19.
- Cohen MV, Yang XM, Downey JM. Smaller infarct after preconditioning does not predict extent of early functional improvement of reperfused heart. Am J Physiol. 1999; 277: H1754-H1761.
- Qiu Y, Tang XL, Park SW, et al. The early and late phases of ischemic preconditioning. A comparative analysis of their effects on infarct size, myocardial stunning, and arrhythmias in conscious pigs undergoing a 40-minute coronary occlusion. Circ Res. 1997; 80: 730-742.
- Sunderdiek U, Schmitz-Spanke S, Korbmacher B, et al. Preconditioning: myocardial function and energetics during coronary hypoperfusion and reperfusion. Ann Thorac Surg. 2002; 74: 2147-2155.
- Jenkins DP, Pugsley WB, Yellon DM. Ischaemic preconditioning in a model of global ischaemia: infarct size limitation, but no reduction of stunning. J Mol Cell Cardiol. 1995; 27: 1623-1632.
- Cohen MV, Liu GS, Downey JM. Preconditioning causes improved wall motion as well as smaller infarcts after transient coronary occlusion in rabbits. Circulation. 1991; 84: 341-349.
- Erb TO, Craig DM, Gaskin PM, et al. Preload recruitable stroke work relationship in the right ventricle: simultaneous assessment using conductance catheter and sonomicrometry. Crit Care Med. 2002; 30: 2535-2541.
- Applegate RJ, Cheng CP, Little WC. Simultaneous conductance catheter and dimension assessment of left ventricle volume in the intact animal. Circ. 1990; 81: 638-648.
- Burkhoff D. The conductance method of left ventricular volume estimation: Methodologic limitations put into perspective. Circ. 1990; 81: 703-706.
- Clark JE, Marber MS. Advancements in pressure-volume catheter technology - stress remodelling after infarction. Exp Physiol. 2013; 98: 614-621.
- Sagawa K. The ventricular pressure-volume diagram revisited.Circ Res. 1978; 43: 677-687.
- Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res. 1974; 35: 117-126.
- Mehmel HC, Stockins B, Ruffmann K, et al. The linearity of the end-systolic pressure-volume relationship in man and its sensitivity for assessment of left ventricular function. Circulation. 1981; 63: 1216-1222.
- Sagawa K. The end-systolic pressure-volume relation of the ventricle: definition, modifications and clinical use. Circulation. 1981; 63: 1223-1227.
- Little WC, Freeman GL. Description of LV pressure-volume relations by time-varying elastance and source resistance. Am J Physiol. 1987; 253: H83-H90.
- Krosl P, Abel FL. Problems with use of the end systolic pressure-volume slope as an indicator of left ventricular contractility: an alternate method. Shock. 1998; 10: 285-291.
- Winter EM, Grauss RW, Hogers B, et al. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation. 2007; 116: 917-927.
- Yang Z, Berr SS, Gilson WD, et al. Simultaneous evaluation of infarct size and cardiac function in intact mice by contrast- enhanced cardiac magnetic resonance imaging reveals contractile dysfunction in noninfarcted regions early after myocardial infarction. Circulation. 2004; 109: 1161-1167.
- Claessens TE, Rietzschel ER, De Buyzere ML, et al. Noninvasive assessment of left ventricular and myocardial contractility in middle-aged men and women: disparate evolution above the age of 50?. Am J Physiol Heart Circ Physiol. 2007; 292: H856-H865.
- Miki T, Cohen MV, Downey JM. Opioid receptor contributes to ischemic preconditioning through protein kinase C activation in rabbits. Mol Cell Biochem. 1998; 186: 3-12.
- Kikuchi C, Dosenovic S, Bienengraeber M. Anaesthetics as cardioprotectants: translatability and mechanism. Br J Pharmacol. 2015; 172: 2051-2061.
- Gross ER, Peart JN, Hsu AK, et al. Extending the cardioprotective window using a novel delta-opioid agonist fentanyl isothiocyanate via the PI3-kinase pathway. Am J Physiol Heart Circ Physiol. 2005; 288: h3744-h3749.
- Meybohm P, Gruenewald M,Albrecht M, et al. Pharmacological postconditioning with sevoflurane after cardiopulmonary resuscitation reduces myocardial dysfunction. Crit Care. 2011; 15: R241.
- Knapp J, Bergmann G, Bruckner T, et al. Pre- and postconditioning effect of Sevoflurane on myocardial dysfunction after cardiopulmonary resuscitation in rats. Resuscitation. 2013; 84: 1450-1455.