Effect of Physiologic Insulin Resensitization on Stages of Chronic Kidney Disease
Author(s): Zachary Villaverde1,2*, Brian Loveridge2, Frank Greenway3, Stanley T. Lewis4, Jonathan RT Lakey5, Roy H. Hinman II1,2, and Richard M. Grimes6
1Island Doctors MSO, St. Augustine, Florida, USA.
2Well Cell Global, Houston, Texas, USA.
3Pennington Biomedical Research Center at Louisiana State University, Baton Rouge, Louisiana, USA.
4Eselle Heath, La Jolla, California, USA.
5Department of Surgery, Department of Biomedical Engineering, University of California Irvine, California, USA.
6McGovern Medical School of the University of Texas at Houston, Department of Internal Medicine, Houston, Texas, USA.
*Correspondence:
Zachary Villaverde, Island Doctors MSO, St. Augustine, Florida, USA, Tel: +1 (904) 614-9998.
Received: 30 Sep 2024 Accepted: 13 Oct 2024
Citation: Zachary Villaverde, Brian Loveridge, Frank Greenway, et al. Effect of Physiologic Insulin Resensitization on Stages of Chronic Kidney Disease. Diabetes Complications. 2024; 8(4); 1-5.
Abstract
Chronic kidney disease (CKD) is common in diabetics. It is usually diagnosed after it has become symptomatic. Treatments of diabetes and CKD are for retarding the progress of the diseases. Reversal of established CKD seldom occurs. This paper presents the study of a treatment that reversed or stabilized CKD in diabetic patients. Twentyone diabetic patients with stages 3a,3b,4 and 5 CKD were treated with Physiologic Insulin Resensitiza-tion (PIR). The peak CKD stages prior to PIR treatment were determined and compared CKD stages at initia-tion of PIR. The Initial CKD stage at initiation of PIR was compared to the stage at the study end. Before starting with PIR. Twelve patient’s peak stage had worsened, 8 were stable and 1 improved. Of the PIR treated patients, 9 improved their stage, 10 were stable and 2 worsened over an average of 18 months. One of the stage 5 patients did not progress to needing dialysis after ten months. Patients with stages 3a, 3b, or 4 seldom reverse their CKD stages. It is remarkable that nine of 21 patients with CKD stages 3a, 3b and 4 improved their stages even though seven of them had a recent history of declining stages. The results have economic implications. As patient’s CKD worsens, treatment cost increases. Preventing stage 4 and 5 patients from progressing to needing dialysis or transplantation could result in hundreds of thousands of dollars in health savings.
Keywords
Introduction
The Centers for Disease Control and Prevention estimates that 38 million Americans or 11.6% of the US popu-lation have diabetes. Of these, 20% are undiagnosed [1]. It is also estimated that 97.6 million people aged 18 years or older have prediabetes (38.0% of the adult US population). This includes 27.2 million people or 48% of those 65 years and over [2]. It is estimated that over one third of persons 65 years and over have chronic kid-ney disease (CKD) [3]. While it is possible for some persons with stage one or stage two CKD to reverse their CKD through diet and exercise. This is rare due to the fact that 90% of those with CKD are not diagnosed until they become symptomatic [2]. Diabetes is a condition which worsens over time. While the decline can be slowed, it is neither curable nor reversible [4]. Among Adults in the United States experiencing kidney failure requiring dialysis or kidney transplant, 47% had diabetes as their primary diagnosis [5]. The number of persons with diabetes needing dialysis and/or kidney transplantation are likely to increase in the future because of the 97.6 million persons with prediabetes. The cost of caring for those with diabetes and CKD is significant. Medi-care recipients who have both diabetes and kidney disease cost twice as much to care for than those without kidney disease. These individuals accounted for 23.5% of Medicare’s fee-for-service expenditures [6].
A different approach to the treatment of Diabetes has shown promise of reversing some of the conditions that are associated with Diabetes. It is called Physiologic Insulin Resensitization (PIR). It mimics the physiologic action of the pancreas in healthy individuals without diabetes. The pancreas in non-diabetics secretes a bolus of insulin every 5-8 minutes, followed by a rest period that allows for insulin receptors on the cells to reset. This causes the body to have sharp peaks and depressions of insulin in blood. In diabetics, the release of insulin is irregular with a constant basal blood insulin, and is the primary driver of insulin resistance. This can exacerbate diabetes and accompanying complications, such as chronic kidney disease. PIR mimics the physiologic release of insulin by intravenously infusing insulin every 5-8 minutes. Blood glucose is closely monitored and is normalized during treatment by oral administration of glucose. The treatments last 3-4 hours and a treatment care plan begins with treatments 2 times per week and transition to weekly, biweekly or monthly depending on the severity of the diabetes and patients’ responses to treatment. A more complete description of the mechanisms involved in this treatment are discussed in papers by Greenway et al and Lewis et al. [7,8].
A pilot study of T2DM patients receiving PIR over 5-6 months has shown favorable changes in markers of chronic kidney disease in 3 patients who experienced an increase in eGFR of 22, 12 and 20 cc/ minute. There-fore, a larger study of patients with type 2 diabetes was conducted to determine whether this study’s findings could be replicated in a larger sample over a longer time frame [9].
Methods
This study was conducted at Island Doctors in Florida, a medical care provider with over 40 locations in Florida. The patients in this study were recruited from 7 different primary care clinics within Island Doctors’ network. These patients attended the two PIR sites near where they received their diabetes care. All patients met the in- clusion criteria of: 1) Having been under the care of Island doctors prior receiving PIR and having estimated glomerular filtration rate (eGFR) assays prior to starting PIR, 2) Having received PIR for seven or more months during the study. and 3) diagnosed with type 2 diabetes and with CKD stages 3a, 3b, 4 or 5 at the time of start-ing PIR. These stages used the conventional classification of eGFRs when 2 = 60-89, 3a = 45-59, 3b = 30-44, 4 = 15-29 and 5 = < 15.
The study was done in two phases. The first phase examined the trajectory of the patients’ eGFRs prior to starting PIR. The highest value during the pre-PIR period was compared to eGFR at the time of starting PIR. Based on the differences in the eGFRS the patients were classified as stable, declining, or improved based on whether there were changes in the stage of their CKD.
The second phase of the study examined the changes the CKD stages after starting PIR. This was done by comparing the eGFR at the start of PIR and the eGFR closest the to the cutoff date of 12/31/22. Before any patient began treatment with PIR at any of the Island Doctors PIR clinic locations, the patient signed a consent document allowing for the anonymous collection of their medical data for the purpose of studies and publications. All data for this study was collected in compliance with the informed consent document completed by the patients. All data were collected through a chart review and, therefore are not subject to IRB review.
Results
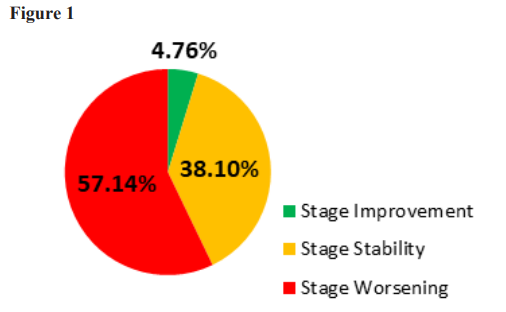
Twenty-one patients met the inclusion criteria of having at least 2 recorded eGFR values documenting their CKD stage prior to beginning PIR, as well as having been treated with PIR for at least 7 months. The number of months included in the pre-PIR phase varied from 3 to 30 months and was due to variations in the times that patients were under the care of Island Doctors or since they were diagnosed with CKD. The highest eGFRs during the period prior to starting PIR revealed that five patients had eGFRs that were stage 2, six were stage 3a, seven were stage 3b, two were stage 4 and one was stage five. The stage 5 patient (patient 21) was on active dialysis before beginning PIR treatment as well as during the course of treatment (See Table 1). The eGFRs measured at the time of starting PIR determined whether the patients’ GFRs were classified as stable, worsening or improving depending on whether they had changed their stage of CKD during the pre-study PIR treatment. The data showed that compared to the peak value before assignment to PIR and the eGFR the value at the start of PIR that, of the 5 pre-PIR stage 2 patients, four had worsened to 3a and one to stage 3b. Of the pre-PIR stage 3a patients, three were stable and three worsened to 3b. Of the seven pre-PIR 3b patients one improved to stage 3a. three were stable and three had worsened to stage 4. One pre-PIR stage 4 patient worsened to stage five and one was stable. The stage 5 pre-PIR patient was stable. In summary, of the 21 patients, 12 (57.1%) worsened, 8 (38%) were stable, and one improved in their CKD stage. (See Table 1 and Figure 1). At the initiation of PIR treatment after the pre-PIR evaluation period, the 21 patients in this study presented with the following break- down of CKD stages: eight stage 3a, seven stage 3b, four stage 4, and two stage 5 (See Table 1).
The time patients were on PIR varied from 7 to 28 months due to patients beginning PIR on a continuing basis in the study period time frame with individualized treatment plans based on their response to PIR. Typically, patients initially underwent infusions twice a week lasting 3 hours for several weeks, followed by weekly infu-sion for 3 months. They then transitioned to weekly, biweekly, or monthly based on the patient response and overall medical condition. After at least 6 months of treatment, three stage 3a patients had stable eGFR levels, three improved and two worsened. One of the latter patients had improved from 3b to 3a prior to initiating PIR and reverted to 3b after receiving PIR. Based on percentage, 38% of the CKD 3a patients in the study showed a full stage improvement to stage 2 after PIR treatment, and 75% either showed a stage improvement or stability at 3a.
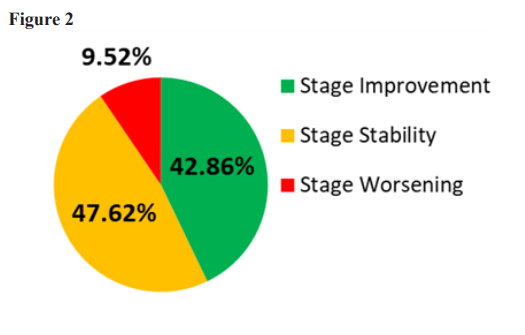
In the seven patients whose beginning PIR was at CKD stage 3b, five (71%) showed a CKD stage improvement, and all seven patients either improved a stage or did not worsen. Likewise, all four stage 4 patients did not worsen over their treatment period, and one of those four transitioned upward to stage 3b. One of the stage 5 patients became stable after declining prior to PIR, and the other remained stable throughout the pre-treatment and PIR treatment periods. Looking at the entire study group, of the twelve patients who had declining eGFR prior to PIR, seven had improvements in their eGFR and five were stable. Of the eight who were stable prior to PIR two improved and five remained stable. One who had been stable declined. In the cohort of 21 patients, after all patients received PIR for a minimum of six months, the percentage of patients whose eGFR stages were worsening decreased from 57% to less than 10%. The percentage of patients remaining stable in their stage increased from 38% to 52%, and the percentage of patients with an increase in eGFR stage increased from 5% to 43% (See Table 2 and Figure 2). Interestingly, the patients with more advanced CKD (stages 3b, 4, and 5) appeared to have a higher percentage of eGFR stability and improvement compared to the entire cohort. In this group, none of the patients had a worsening decline in renal function after beginning PIR, and of the patients that were showing progressive decline, over 60% of them either reversed their downward progression or showed a rise in eGFR and were reclassified to higher stages of CKD. One patient’s final eGFR measurement improved two stages (from 3b to 2).
Discussion
Prior to receiving PIR, the patients’ CKD stage behaved as expected with a majority declining while all of the rest but one remained stable. However, it is unexpected that, after receiving PIR, 9 of 21 CKD patients have reversed their CKD stages during a period that averaged 18 months. In addition, 10 of the other 12 patients’ CKD stages remained stable. These changes are remarkable when one considers that 12 of the 19 improving or stabilized patents had a history of worsening CKD stages prior to receiving PIR [10]. It is remarkable to find that 9 of 21 diabetic patients increased their CKD stages over periods that averaged 16 months. This is particularly unusual when one considers that 7 of the 9 had a recent history of declining CKD stages over an average of 14 months prior to receiving PIR. It is also notable that 10 of 21 patients remained stable during periods ranging from 10 to 28 months. It is Particularly noteworthy that, of the patients who were classified as stage four or five, remained stable from 10 to 28 months except for one stage four who improved to stage 3a. A 2017 study by Caravaca-Fontan et al. followed CKD 4 and 5 patients over the progression of their disease 16 months. They reported that 64% of these patients were on active dialysis, and 16% of the total cohort had died [11]. Except for the one patient who was on dialysis prior to going on PIR, none of the CKD 4 and 5 patients in this study showed any decline in eGFR and none had worsened to the state where dialysis or transplantation would be considered essential. These results can have significant cost implications given that the costs of transplantation and dialysis are so high. Bentley and Ortner estimated the average charges for kidney transplants, including charges for 30 days pre-surgical services, organ procurement, hospitalization, and 180 days post hospitalization costs were $442,000 [12]. Kidney dialysis costs are also significant. Kaplan et al. reported that the cost to Medicare of kidney dialysis ranged from $91,716 to $108,656 per year [13]. This small, retrospective review is a starting point for rigorous prospective studies to determine if adding PIR to the current standard of care would be a material advance in treating this condition. Additional metrics to determine kidney function, such as Urine Albumin/Creatinine Ratio (UACR), will need to be captured in these future studies for even more comprehensive results in future.
Acknowledgments
The authors wish to thank and acknowledge the clinical and administrative staff at Island Doctors for their assistance in patient care and data collection, Michael Alexander at University of California Irvine for his assistance in manuscript review and data analysis, as well as Eric Dyess, MD, FACE, and Leah Amir, MS, MHA for their review and commentary on this manuscript.
References
- Centers for Disease Control and Prevention. A Report Card: Diabetes In the United States. 2024. https://www.cdc.gov/diabetes/communication-resources/diabetes-statistics.html
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States Centers for Disease Control and Prevention US Department of Health and Human Services. 2023. https://nccd.cdc.gov/CKD/Documents/Chronic-Kidney-Disease-in-the-US-2023-h.pdf
- US Department of Health and Human Services, NIDDK USRDS annual 2022. https://usrds-adr.niddk.nih.gov/2022/chronic-kidney-disease/1-ckd-in-the-general-population
- Centers for Disease Control and Live well with chronic kidney disease. 2022. https://www.cdc.gov/kidneydisease/publications-resources/live-well-with-chronic-kidney-disease
- National Kidney Kidney disease the basics. 2024. https://www.kidney.org/news/newsroom/fsindex#how-many-people-require-dialysis-or-transplant
- National Institute of Diabetes and Digestive and Kidney Healthcare expenditures for persons with CKD. In Annual Data Report. Chapter 6. 2022. https://usrds-adr.niddk.nih.gov/2022/chronic-kidney-disease/6-healthcare-expenditures-for-persons-with-ckd
- Greenway F, Loveridge B, Grimes RM, et al. Physiologic insulin resensitization as a treatment modality for insulin resistance Int J Mol. 2022; 23: 1884.
- Lewis ST, Greenway F, Tucker TR, et al. A Receptor Story Insulin Resistance Pathophysiology and Physiologic Insulin Resensitization's Role as a Treatment Int J Mol Sci. 2023; 24: 10927.
- Villaverde Z, Tucker T, Alexander M, et Improved kidney function following physiologic insulin resensitization treatment modality. Endocrinology and Disorders. 2021; 5: 80.
- National Institute of Diabetes and Digestive and Kidney United States Renal Data System progress through research 2022. In Annual Data Report. Chapter 6. 2023. https://usrds-adr.niddk.nih.gov/2022/chronic-kidney-disease/6-healthcare-expenditures-for-persons-with-ckd
- Caravaca-Fontán F, Lilia Azevedo, Enrique Luna, et Patterns of progression of chronic kidney disease at later stages. Clinical Kidney Journal. 2018; 11: 246-253.
- Bentley TS, Ortner 2020 US organ and tissue transplants: Cost estimates, discussion and emerging issues. 2020. https://www.milliman.com/-/media/milliman/pdfs/articles/2020-us-organ-tissue-transplants.ashx
- Kaplan JM, Niu J, Ho V, et A comparison of US Medicare expenditures for hemodialysis and peritoneal dialysis. J Am Soc Nephrol. 2022; 33: 2059-2070.