Effects of Light Therapy on Vascular Function in Patients with Diabetic Peripheral Neuropathy
Author'(s): Arturo A. Arce-Esquivel1*, Gloria Duke2, Jody K. Takemoto3, Carol A. Rizer2, and Joyce E. Ballard1
1 Department of Health and Kinesiology, College of Nursing and Health Sciences, The University of Texas at Tyler, Tyler, Texas, United States
2 School of Nursing, College of Nursing and Health Sciences, The University of Texas at Tyler, Tyler, Texas, United States
3 Department of Pharmaceutical Sciences, Ben and Maytee Fisch College of Pharmacy, The University of Texas at Tyler, Tyler, Texas, United States
*Correspondence:
Arturo A. Arce-Esquivel, Department of Health and Kinesiology, College of Nursing and Health Sciences, The University of Texas at Tyler, Texas, United States, Tel: (903) 565-5838; Fax: (903) 566-7065; E-mail: aarce@uttyler.edu
Received: 23 July 2018; Accepted: 31 August 2018
Citation: Arturo A. Arce-Esquivel, Gloria Duke, Jody K. Takemoto, et al. Effects of Light Therapy on Vascular Function in Patients with Diabetic Peripheral Neuropathy. Cardiol Vasc Res. 2018; 2(3): 1-6.
Abstract
Diminished vascular function has been reported to be common in patients with diabetic peripheral neuropathy (DPN) and acts as a major contributor to cardiovascular disease as well as lower limb complications. DPN is a consequence of diabetes-mediated impairment blood flow which leads to microvascular disturbances, which is best characterized as neuropathic pain. Foot pain due to DPN is one of the factors affecting walking ability. Infrared light therapy has been shown to be effective in reducing pain and increasing local circulation in a variety of painful conditions including neuropathy. This study aimed to determine the effects of infrared light therapy treatment on vascular function (i.e., microcirculation) and pain relief among patients with DPN. Nine patients (age: 74 ± 8.68 years) participated in this study. Infrared light therapy treatment was applied 3 times per week for 30 minutes per day, across 5 weeks. The light therapy intervention was performed using the Anodyne Therapy System (ATS). Before and after infrared light therapy treatment, vascular function [Digital Thermal Monitoring (DTM) of vascular reactivity] and pain assessment [Brief Pain Inventory-short form (BPI-SF) and short-form McGill Pain Questionnaire (SF-MPQ)] were evaluated. After the 5-week treatment, the vascular reactivity index (i.e., microvascular function) increased significantly by 25% from baseline (1.76 ± 0.13 to 2.20 ± 0.15, p < .05). In addition, the BPI-SF and SF-MPQ showed that pain decreased following light therapy. Our preliminary findings indicated that infrared light therapy promoted positive effects on microvascular function and pain relief in patients with DPN.
Keywords
Introduction
In peripheral neuropathy with a definable cause, type 2 diabetes mellitus (T2DM) is the most commonly seen worldwide [1]. Indeed, diabetic peripheral neuropathy (DPN), a well-known, long-term complication of T2DM, can affect almost half of the diabetic population and is associated with higher morbidity and mortality [2]. DPN encompasses a variety of clinical or subclinical presentations. The early stages of DPN are often characterized by pain and tingling. Painful DPN is a common type of diabetic neuropathy and the most common cause of neuropathic pain [2]. DPN symptoms exhibit a symmetrical “stocking and gloves” distribution and are often associated with nocturnal exacerbation. It can be presented from a mild pins and needles sensation to stabbing, burning, unremitting, or even unpleasant electric shock sensation [1,3]. Foot pain due to DPN is one of the factors that prevents patients from performing their activities of daily living (ADLs), such as walking ability [3].
DPN, a progressive disease, is a consequence of diabetes-mediated impairment in blood flow to, and resultant hypoxia of, nerves [4]. Indeed, the pathological changes associated with the disease are in many cases consistent with ischemia, secondary to vascular insufficiencies [5]. The literature has clearly established that chronic ischemia contributes to demyelination or axonal degeneration [6]. The reduction of nerve conduction velocity in DPN has been shown to be preceded by impaired vasodilation of the arterioles [7]. Microvascular dysfunction is common in DPN and acts as a major contributor to cardiovascular disease as well as lower limb complications [8]. Clearly, these findings suggest that signs of microvascular disease are present prior to the development of clinically overt DPN. Due to the increasing prevalence of TD2 the Neuropathy Association estimates that there are now 15-18 million Americans with DPN [9]. This growing DPN statistic significantly raises the overall number of people with neuropathy in the U.S. Indeed, recent projections estimate that by 2050, as many as one in every three Americans will have T2DM [10]. Therefore, the reduction of foot pain can likely allow patients with DPN to walk easier, and hence significantly improve their overall well-being.
Interestingly, infrared light therapy, a non-invasive FDA approved treatment has been shown to be effective in a variety of painful conditions including back pain [11], knee pain [12], and shoulder tendinopathies [13]. In addition, there is evidence that light therapy improves sensation and might reduce pain in the feet of patients with DPN [14,15]. However, in those studies pain was assessed using a screening tool designed to determine overall neuropathic symptoms [16]. The probable mechanism of action underlying pain relief associated with infrared light therapy may be due, in part, to a combination of topical heat and increased circulation as a result of dilating arteries and veins. Therefore, improving blood flow in the feet of patients with DPN could help restore sensation and promote pain relief. Furthermore, restoration of adequate circulation may reverse neuropathy and thus delay the onset of ulcerations that often lead to amputation. We are unaware of any study that has examined the effect of light therapy on peripheral microcirculation. Thus, the primary aim of this preliminary study was to determine the effects of infrared light therapy treatment on vascular function (i.e., microcirculation) and the associated pain relief among patients with DPN.
Methods
Study participants
Nine patients (men, n = 5, women, n = 4) with a physician’s diagnosis of DPN were recruited from the East Texas area via a local newspaper advertisement to participate in this study. The number of participants was limited to the number who could be safely accommodated in the space available at the university. The inclusion criteria for the study were (a) ability to maintain an upright posture for at least one minute voluntarily, (b) ability to walk at least 20 yards independently, (c) willingness to participate in the study, and (d) a diagnosis of bilateral PN. Individuals were excluded from participation if there was (a) neurological disorder unrelated to DPN, (b) pain conditions that may confound PN pain assessment, (c) PN confined only to upper extremities, (d) asymmetric symptoms of PN, (e) history of drug/alcohol abuse, (f) amputation other than toes, and (g) skin conditions altering sensation. Following explanation of all the details of the study, each participant signed an informed consent approved by the Institutional Review Board. Following consent, physician’s approval and a completed health history from the physician’s office were required before the intervention began.
Experimental design
This study utilized a controlled experimental design to determine the effectiveness of a 5-week infrared light therapy treatment. The Health and Physical Activity Questionnaire [17] was used to determine the level of physical activity behavior, and the medical history of each patient before the study started. Each participant was examined during two separate visits (i.e., before and after the 5-week treatment) for the measurement of both vascular function and pain assessment.
Vascular function
The studies were conducted after an overnight fast of at least 10 hours (water was permitted) and abstinence from tobacco, alcohol, caffeine, vasoactive medications, exercise, high-fat foods, and vitamin C ingestion. The measurements were obtained after the patients were seated for 20 minutes of rest. The fingertip Digital Thermal Monitoring (DTM), a non-invasive test, of vascular reactivity (i.e., microvascular function) was used [18]. All DTM measurements were performed in a quiet, dimly lit room with a controlled ambient temperature between 23 and 25°C. Segmental pressure cuffs were positioned around the forearms. Disposable finger probes were attached to the index fingers of both hands. DTM of both hands was obtained during 5-minute stabilization, 5-minute cuff inflation, and 5-minute deflation using an automated, operator independent protocol (VENDYS, Endothelix Inc., Houston, TX, USA). The right upper arm cuff (i.e., occluded arm) was rapidly inflated to 50 mmHg above systolic pressure for 5 minutes and then rapidly deflated to invoke reactive hyperemia distally. Thermal changes during a 5-minute arm-cuff-induced reactive hyperemia test were monitored continuously in the fingertip of both the occluded (i.e., right) and the non-occluded (i.e., left) arms using VENDYS software. Finally, the area under the temperature curve was used to determine the vascular reactivity index (VRI) that assessed the difference in response in microvascular function before and after light therapy treatment.
Pain assessment
Because pain has multifaceted, subjective and complex features, it can be difficult to measure and caution must be used in selecting valid and reliable instruments [19]. Hence, we selected two appropriate screening instruments, the Brief Pain Inventory-short form (BPI-SF) and the short-form McGill Pain Questionnaire (SFMPQ). Both, the BPI-SF and the SF-MPQ, are well-known for their sensitivity and reliability [20,21]. For instance, the BPI-SF has been shown to have strong psychometrics and criterion validity in studies by Kim et al., (Cronbach’s reliability; 0.9 for severity and 0.94 for interference) [21] and Zelman et al., (Cronbach’s reliability; 0.94 for both scales) [20] among patients with DPN. In addition, the BPI-SF is an important tool that determines pain severity and impact on ADLs [22]. The numerical rating scale has three items that measure severity while seven items assess the degree that pain interferes with ADLs. The patients were asked to rate their worst, least, average, and current pain intensity, to list current treatments and their perceived effectiveness, and to rate the degree that pain interfered with general activity, mood, walking ability, normal work, relations with other persons, sleep, and enjoyment of life on a 10-point scale. Possible ranges of scores for pain in the previous 24 hours were from “no pain” (=0) to “pain as bad as can be imagined” (=10). The probable scores for pain interfering with certain ADLs ranged from “no interference” (=0) to “completely interfere” (=10).
The SF-MPQ [23] provides a more comprehensive pain evaluation (i.e., pain rating, present pain intensity, and pain over previous week). The SF-MFQ contains (a) 15 items in pain rating index or PRI (11 are sensory/pain descriptor items and 4 are affective descriptor items), (b) 1 item measuring present pain intensity or PPI, and (c) 1 item rating pain over previous week using a visual analog scale (VAS). Briefly, each item in the PRI is rated on a 3-point scale from “mild” (=1) to “severe” (=3). Participants were instructed to not mark any descriptor if there was no pain or affective response, and these were rated during analysis as absent (=0). The VAS consists of one 10 cm horizontal line that is anchored with verbal descriptors of “no pain” (=0) and “worst possible pain” (=10). VAS for pain ratings have been shown to be more sensitive for chronic pain measurement and for detecting changes [19,24] and recommended for studies measuring pain outcomes as a result of interventions [25]. Finally, scores for the SF-MFQ can range from 0 to 45 on the PRI, from 0 to 5 on the PPI, and from 0 to 10 cm on the VAS; with a [lower/higher] score indicating [more/less] pain experienced.
The patients completed both; the BPI-SF and the SF-MPQ; instruments alone or with assistance from the researchers and trained research assistants. Assistance was needed when vision was impaired and/or patients were unable to clearly decipher the pain tool items. Tools were checked for all items being addressed following participant completion.
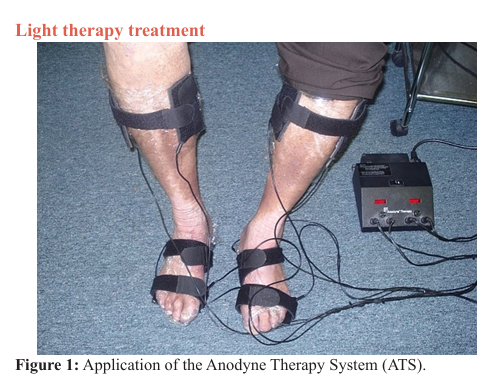
The infrared light therapy treatment was applied to the feet and legs of study participants (Figure 1), offered 3 times per week for 30 minutes per day, over 5 weeks. The light therapy intervention was performed using the Anodyne Therapy System (ATS) Model 480 (Anodyne Therapeutics LLC, Tampa, Florida). The ATS is a medical device consisting of a base power unit and therapy pads containing 60 near-infrared (890 nm) gallium aluminum arsinide diodes used to increase circulation by dilating arteries and veins. Each ATS unit has eight flexible therapy pads (4 per limb). The flexible therapy pads were placed in contact with each patient’s skin during the treatment period as shown in Figure 1. Specifically, one therapy pad was placed on the dorsal and one on the plantar aspect of the foot as well as one on the lateral and one on the medial aspect of both lower extremities, immediately above the ankle in each patient.
Statistical analysis
All values are means ± standard deviation (SD). The Wilcoxon Matched-Pairs Signed-Ranks test was used to determine significant differences between the two data collection points. This nonparametric test is used instead of paired t-tests when there is nonnormal distribution of the data for determining differences between two sets of scores from the same participants. All statistical analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL, USA). The level of significance for all analyses was set at p ≤ .05.
Results
Participant characteristics
A total of 9 patients (age: 74 ± 8.68 years) with bilateral DPN participated in all aspects of this study. The patients’ characteristics are presented in Table 1. Forty-four percent of the patients were women, and 56% men. The patients ranged in age from 56 – 82 years. Eighty-nine percent of the participants were over the age of 65. The physical activity behavior indicated that these patients were low-to-moderately active. The length of time that patients held the diagnosis of DPN ranged from 3 to 20 years, with 70% of the participants experiencing DPN for over 10 years. Forty four percent of the patients had a BMI greater than 30kg•m-2. Finally, all the patients completed the 5-week infrared light therapy treatment (i.e., 15 sessions in total). No adverse reactions to the treatment were reported.
Vascular function
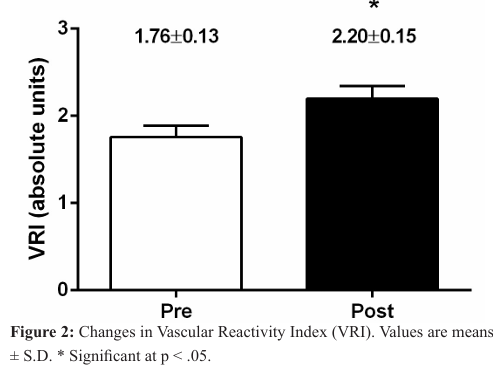
The average values for the vascular function assessments are presented in Figure 2. The average baseline VRI was 1.76 ± 0.13. Following the 5-week infrared light therapy treatment, the VRI (i.e., microvascular function) increased significantly by 25% from baseline (1.76 ± 0.13 to 2.20 ± 0.15, p < .05).
Pain assessment
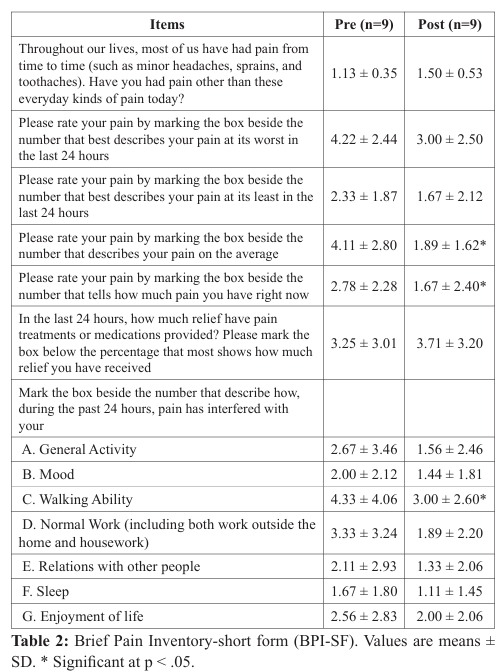
The average values for the BPI-SF are presented in Table 2. Following the 5-week infrared light therapy treatment, the BPI-SF scores decreased significantly in three items; (a) average pain (4.11 ± 2.80 to 1.89 ± 1.62; p =.026), (b) current pain or pain felt right now (2.78 ± 2.28 to 1.67 ± 2.40; p = .026), and (c) the degree that pain interfered with walking ability (4.33 ± 4.06 to 3.00 ± 2.60; p = .042). There were not significant differences among the other items assessed by the BPI-SF.
In the SF-MPQ, two items that assessed level of pain decreased significantly after the 5-week infrared light therapy treatment. These items were “stabbing” (0.78 ± 0.83 to 0.33 ± 0.50; p = .044) and “hot burning” pain (0.89 ± 1.05 to 0.33 ± 0.71; p = .025). Most of the other pain characteristics ranged from absence to moderate with only one reporting severe for cramping pain, one for hot burning pain, one for tiring-exhausting pain, and one reported fearful pain.
Discussion
This preliminary study was aimed to assess the effects of infrared light therapy treatment on vascular function (i.e., microcirculation) and pain relief among patients with DPN. Our findings revealed that infrared light therapy promoted positives effects on microvascular function and pain relief.
Vascular function
The DTM is a non-invasive technique to study vascular reactivity (i.e., microvascular function) in humans. The repeatability of DTM is excellent and can be used as a reproducible and operator-independent test for non-invasive measurement of vascular function [18,26,27]. Indeed, DTM of vascular reactivity appears to be an appropriate test to evaluate vascular reactivity in clinical and research settings [18,26]. Briefly, a standard armcuff promotes a temporary occlusion of blood flow in the arm. During the cuff occlusion, the lack of blood flow (i.e., ischemia) elicits a microvascular dilative response. Upon releasing the cuff, blood flow rushes into the forearm and hand, not only restoring baseline flow but also resulting in an overshoot (i.e., reactive hyperemia). The reactive hyperemia promotes shear stress in the larger arteries to dilate and accommodate the increased blood flow. In general, the study of blood flow responses following occlusion indicates that younger, fitter, and healthier individuals exhibit greater flow responses, suggesting better vascular function. Reductions in the hyperemic response, a hallmark feature of impaired vascular function, are observed during DTM. The VRI provides a non-invasive “window” to the cardiovascular system allowing the detection, measurement, and monitoring of vital patient information such as overall cardiovascular health. The VRI is reported in absolute units using a vascular reactivity scale: a) < 1 (“poor”), b) 1 - 2 (“intermediate”), and c) > 2 (“good”) (VENDYS, Endothelix Inc., Houston, TX, USA).
The average baseline (i.e., before light therapy) VRI was 1.76 ± 0.13 (Figure 2). These vascular assessments placed the participants in the “intermediate” category of vascular reactivity (1-2) at the beginning of the intervention. Similar baseline VRI values have been reported previously, by our laboratory, among patients with PN [28]. Together, these findings suggest that microvascular function in individuals with PN is compromised. Such a compromised vascular function could contribute to the progression of the disease. In fact, the literature provides significant evidence that conditions of acute and chronic ischemia contribute to changes in peripheral nerve function and structure [6]. Previous studies have reported a strong correlation between severity of nerve damage and stage of vascular insufficiency [6,8]. There are also reports of reduced vasodilation in response to heat and iontophoresis of acetylcholine in the presence of DPN [29,30].
Importantly, our findings report that following a 5-week infrared light therapy treatment, the VRI increased significantly (25% increase from baseline, 1.76 ± 0.13 to 2.20 ± 0.15. p < .05) (Figure 2). To our knowledge, this is the first study that has shown infrared light therapy was able to improve peripheral microcirculation among patients with DPN. We speculated that the increased microvascular reactivity (i.e., increased blood flow), observed in our study, was mediated by a combination of metabolic and endothelial factors (e.g., nitric oxide, a potent vasodilator).
Pain relief
Previous studies have reported temporary increases in foot sensitivity following the application of infrared light therapy in patients with diabetic or nondiabetic PN [14,15]. For instance, the cohort by Harkless et al., reported improved foot sensation as well as a reduction in neuropathic pain after light therapy treatments in 2239 community-dwelling patients with PN, 62% with DPN [15]. However, it is important to mention that the cohort by Harkless et al. [15] evaluated neuropathic pain using only the VAS; which is only one of the different items included in the SF-MPQ screening tool [23] used in our study. In addition, the study by Leonard et al. [14] also reported that light therapy improved foot sensation and reduced pain in patients with DPN. These investigators found that the patients’ responses to the Michigan Neuropathy Screening Instrument (MNSI) showed that neuropathic symptoms decreased after light therapy [14]. The MNSI questionnaire is a more sensitive screening tool for DPN. The MINSI provides a graded patient response of neuropathic symptoms, and is designed to screen for the presence of DPN [16].
Our study found that infrared light therapy treatment promoted pain relief among patients with DPN using the BPI-SF and SFMPQ. Indeed, pain scores decreased in three areas of the BPI-SF including average pain in the previous 24 hours, current pain, and the degree to which pain interfered with walking in the previous 24 hours (Table 2). These findings are quite relevant considering how pain affects the walking ability and balance in patients with DPN. Clearly, the improvement in walking ability may offer an opportunity for fall-related risk reduction in this population and maintenance of a good quality of life.
These findings are particularly interesting since the existing evidence clearly indicates microvascular and neural complications of diabetes are major contributors to lower limb pathology in DPN [8]. So, it is our hope that these data would stimulate clinicians to consider assessing vascular function in patients with DPN, in an effort to better understand the impact of DPN on microvascular function. We do remain cautious in our interpretations because the lack of a mechanistic approach and the small sample size which prevents more sophisticated speculation regarding the underlying positive effects of infrared light therapy on microvascular function and pain relief.
Conclusion
The present findings demonstrated that in patients with DPN, a 5-week infrared light therapy treatment is capable of increasing microvascular function and providing pain relief. Considering that chronic neuropathic pain and gait problems are factors affecting walking ability in DPN and might have a negative impact on quality of life, treatments like infrared light therapy, might be an effective alternative therapeutic option suitable for this clinical population.
Acknowledgments
The authors gratefully acknowledge the group of undergraduate students for their technical assistance during the light therapy treatment.
References
- Hughes RA. Peripheral neuropathy. BMJ Clinical research ed. 2002; 23: 466-469.
- Aslam A, Singh J, Rajbhandari S. Pathogenesis of painful diabetic neuropathy. Pain research and treatment. 2014; 412041.
- England JD, Asbury AK. Peripheral neuropathy. Lancet. 2004; 26: 2151-2161.
- Tremont-Lukats IW, Megeff C, Backonja MM. Anticonvulsants for neuropathic pain syndromes: mechanisms of action and place in therapy. Drugs. 2000; 60: 1029-1052.
- Krishnan AV, Lin CS, Kiernan MC. Nerve excitability properties in lower-limb motor axons: evidence for a lengthdependent gradient. Muscle & nerve. 2004; 29: 645-655.
- Laghi Pasini F, Pastorelli M, Beermann U, et al. Peripheral neuropathy associated with ischemic vascular disease of the lower limbs. Angiology. 1996; 47: 569-577.
- Coppey LJ, Davidson EP, Dunlap JA, et al. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. International journal of experimental diabetes research. 2000; 1: 131-143.
- Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes/metabolism research and reviews. 2009; 25: 604-614.
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet (2014). Available from: http://www.ninds.nih.gov/disorders/peripheralneuropathy/detail_peripheralneuropathy.htm.
- Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics. 2010; 8: 29.
- Leichtfried V, Matteucci Gothe R, Kantner-Rumplmair W, et al. Short-term effects of bright light therapy in adults with chronic nonspecific back pain: a randomized controlled trial. Pain medicine. 2014; 15: 2003-2012.
- Leal-Junior EC, Johnson DS, Saltmarche A, et al. Adjunctive use of combination of super-pulsed laser and light-emitting diodes phototherapy on nonspecific knee pain: double-blinded randomized placebo-controlled trial. Lasers in medical science. 2014; 29: 1839-1847.
- Montes-Molina R, Martinez-Rodriguez ME, Rodriguez AB, et al. Interferential light therapy in the treatment of shoulder tendinopathies: a randomized controlled pilot study. Clinical rehabilitation. 2012; 26: 1114-1122.
- Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebocontrolled study with monochromatic near-infrared treatment. Diabetes care. 2004; 27: 168-1672.
- Harkless LB, DeLellis S, Carnegie DH, et al. Improved foot sensitivity and pain reduction in patients with peripheral neuropathy after treatment with monochromatic infrared photo energy--MIRE. Journal of diabetes and its complications. 2006; 20: 81-87.
- Feldman EL, Stevens MJ, Thomas PK, et al. A practical twostep quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes care. 1994; 17: 1281-1289.
- Bauman A, Phongsavan P, Schoeppe S, et al. Physical activity measurement--a primer for health promotion. Promotion & education. 2006; 13: 92-103.
- Ahmadi N, McQuilkin GL, Akhtar MW, et al. Reproducibility and variability of digital thermal monitoring of vascular reactivity. Clinical physiology and functional imaging. 2011; 31: 422-428.
- Younger J, McCue R, Mackey S. Pain outcomes: a brief review of instruments and techniques. Current pain and headache reports. 2009; 13: 39-43.
- Zelman DC, Gore M, Dukes E, et al. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. Journal of pain and symptom management. 2005; 29: 401-410.
- Kim SS, Won JC, Kwon HS, et al. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes research and clinical practice. 2014; 103: 522-529.
- Mendoza T, Mayne T, Rublee D, et al. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. European journal of pain. 2006; 10: 353-361.
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987; 30: 191-197.
- Phillips KD, Skelton WD, Hand GA. Effect of acupuncture administered in a group setting on pain and subjective peripheral neuropathy in persons with human immunodeficiency virus disease. Journal of alternative and complementary medicine (New York, NY). 2004; 10: 449-455.
- McGuire DB. Comprehensive and multidimensional assessment and measurement of pain. Journal of pain and symptom management. 1992; 7: 312-329.
- Zeb I, Ahmadi N, Molnar MZ, et al. Association of coronary artery calcium score and vascular dysfunction in longterm hemodialysis patients. Hemodialysis international International Symposium on Home Hemodialysis. 2013; 17: 216-222.
- McQuilkin GL, Panthagani D, Metcalfe RW, et al. Digital thermal monitoring (DTM) of vascular reactivity closely correlates with Doppler flow velocity. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2009; 2009: 11001113.
- Arce-Esquivel AA, Ballard JE, Haas BK, et al. Effect of tai chi on vascular function among patients with peripheral neuropathy. J Heart Cardiol. 2016; 2: 1-6.
- Pfutzner A, Forst T, Engelbach M, Margin T, Goitom K, Lobig M, et al. The influence of isolated small nerve fibre dysfunction on microvascular control in patients with diabetes mellitus. Diabetic medicine: a journal of the British Diabetic Association. 2001; 18: 489-494.
- Arora S, Smakowski P, Frykberg RG, et al. Differences in foot and forearm skin microcirculation in diabetic patients with and without neuropathy. Diabetes care. 1998; 21:1339-1344.