Endocarditis with No Valve Involvement? A Case of Superior Vena Cava Endocarditis, Rare but Serious Vascular Infection
Author'(s): Ziad Affas M.D*, Anuradha Sreenivasan D.O2 and Vijay Patel M.D3
1Internal Medicine Resident, PGY-2, Henry Ford Macomb Hospital / Department of Internal Medicine, Michigan.
2Internal medicine attending at Henry ford Macomb hospital, Clinton Township, Michigan.
*Correspondence:
Ziad Affas M.D, Internal Medicine Resident, PGY-2, Henry Ford Macomb Hospital / Department of Internal Medicine, Michigan
Received: 25 Jul 2022; Accepted: 28 Aug 2022; Published: 03 Sep 2022
Citation: Ziad Affas, Anuradha Sreenivasan D.O, Vijay Patel. Endocarditis with No Valve Involvement? A Case of Superior Vena Cava Endocarditis, Rare but Serious Vascular Infection. Cardiol Vasc Res. 2022; 6(4): 1-3.
Abstract
Patients with end-stage renal disease (ESRD) will likely need vascular access for hemodialysis, the vascular access increases the risk of having bloodstream infections such as Catheter-Related Bloodstream Infections (CRBSI). Rarely some patients may have non-valvular endocarditis. Fibrin sheath usually plays a role in this infection, and these can be identified on a transesophageal echocardiogram (TEE). ESRD patients can present without fever in cases of endocarditis due to uremia impaired cellular host defenses, therefore, a low threshold for TEE should be considered in these patients. We report a case of SVC endovascular endocarditis in the setting of MSSA bacteremia in an ESRD patient.
A 49 -year-old female with a past medical history significant for ESRD secondary to diabetic nephropathy on dialysis with a tunneled subclavian hemodialysis dialysis catheter. Presented to the hospital for a chief complaint of fever for 2 days duration. The patient was vitally stable on admission; the area around the catheter was indurated and tender. The patient had a blood culture on the first day of her fever, it came back positive upon admission as the blood culture showed staphylococcus aureus bacteremia, and the patient was started on IV Vancomycin.
Ultrasound was done and showed an abscess surrounding the dialysis port. TTE was done and showed no obvious evidence of endocarditis. TEE was done which demonstrated a large, mobile RA mass consistent with vegetation, which is attached at the lower junction of SVC and right interatrial septum. Angiovac of vegetation was done. The catheter was out. Blood culture sensitivity grew MSSA, susceptible to ancef, therefore vancomycin was switched to ancef 2g for a total of 6 weeks.
Clinical suspicion should always be high for endocarditis in patients with fever and having dialysis catheter. TTE can be negative many times in these patients; therefore, having a high index of suspicion should always be there in these patients. Non-valvular endocarditis such as superior vena cava endocarditis is very uncommon and is only documented in a few cases in the literature. This infection responds very well to the antibiotic and Angiovac is helpful in these situations too.
Keywords
Introduction
Patients with end-stage renal disease (ESRD) will likely need a vascular access for hemodialysis and 25% of them will have a catheter as the vascular access [1]. This vascular access increases the risk of having blood stream infections, as Catheter Related Bloodstream Infections (CRBSI) is one of the most common causes of bacterial infections in hemodyalsis patients [2]. These healthcare related blood stream infections include sepsicemia and valvular endocarditis. However rarely some patients may have non- valvular endocarditis. Fibrin sheath usually begins to form at the time of the insertion of the catheter [3]. When the endothelium is injured, a pericatheter thrombus forms as early as within 48 hours, organizes into fibroepithelial tissue over the course of several days, and finally creates an endothelial layer indistinguishable from the vein wall at 30 days that remains intact within the lumen of the vein after removal of the catheter [3]. A fibrin sheath is the most common cause of indwelling catheter dysfunction and is primarily described in the setting of catheter occlusion, failure, and vein stenosis [3]. The theory of the vascular endocarditis in these patients is believed to be secondary to firbin sheath vegetation [3], and these can be identified on transesophageal echocardiogram (TEE) [3]. ESRD patients can present without fever in cases of endocarditis due to uremia impaired cellular host defenses, therefore, a low threshold for TEE should be considered in these patients [4]. We report a case of SVC endovascular endocarditis in the setting of MSSA bacteremia in an ESRD patient.
Case Report
A 49 -year-old female with a past medical history significant for ESRD secondary to diabetic nephropathy on dialysis with a tunneled subclavian hemodialysis dialysis catheter, type 2 diabetes without insulin use, hypertension, anemia of chronic disease secondary to ESRD. Presented to the hospital for a cheif complaint of fever for 2 days duration. Patient states that 2 days prior, she received her normal dialysis and immediately started having chills, fever of 101°, fatigue but no redness or swelling were noticed. She stated that blood cultures were taken at that time and she was then placed on a IV antibiotic. However, her condition did not improve so she came to the hospital.
On physical examination, her vital signs showed that her blood pressure was 119/94, pulse rate was 100 beats per minute, temperature of 99.2F. Respiratory rate was 19 breaths per minute, and SpO2 was 100% on room air. Area around the catheter was indurated and tender, however non-erythematous. Physical examination otherwise was unremarkable. By the time the patient reached the hospital, her blood culture came back positive for staphylococcus aureus bacteremia, and the patient was started on IV Vancomycin. Day one of hospitalization.

Ultrasound was done and showed abscess measuring 5.1 x 2.1 x 1.2 cm surrounding the dialysis port. The plan was to put the patient off dialysis for 2 days by this time. Therefore, nephrology decided to do dialysis at that day using that access before it will be taken off, and the plan was for vascular surgeons to remove the cath the day after.
Cardiology was also consulted at that time for transthoracic and transesophageal echocardiography given staphylococcus aureus bacteremia. TTE was done showed no obvious evidence for endocarditis.
Day two of hospitalization, patient continued on IV antibiotics, catheter was removed, blood culture was repeated, patient continued to be afebrile and vitally stable.
Day three of hospitalization, blood culture showed gram positive bacteremia, therefore cardiology and infectious disease agreed on doing TEE, which the patient was scheduled after two days, patient continued to be on IV antibiotics.
Day five of hospitalization, the patient continued to be vitally stable, TEE was done (showed below) which demonstrated a large, mobile RA mass consistent with vegetation which is attached at the lower junction of SVC and right interatrial septum. The mass measured approximately (19.1 mm x 15.5 mm). The patient was transferred to another hospital for the potential possibility of angiovac of vegetation and then dialysis access. Blood culture sensitivity grew MSSA, suspectable to ancef, therefore vancomycin was stopped and IV ancef 2g three times weekly for total of 6 weeks was started.
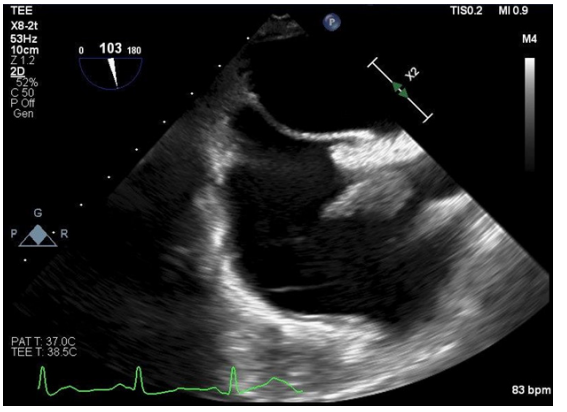
Day six for hospitalization, new left internal jugular tunneled cuffed catheter was placed, and dialysis session was done.
Day seven of hospitalization, patient underwent a successful Angiovac for the SVC/RA junction mass. TEE was repeated on the day after the patient continued to be vitally stable; the patient was discharged to home on IV ancef 2g with instruction to follow up with cardiology and nephrology. The patient followed with both of them and was scheduled for another TEE about 2 months as a follow-up; however, she refused doing the test. The patient continued to be stable during the whole time, her WBC went down to 4.5 K/ul, blood culture was after one month of the treatment and when she finished her 6 weeks treatment and it was negative in both occasions.
Discussion
Patients, such as the one in this case, who are on HD have a significantly higher incidence of IE compared to the general population. Although left sided endocarditis is being the most common type of endocarditis, 10% of endocarditis can be on the right side and mainly in intravenous drug abusers, intracardiac devices, and central venous catheters [5]. Right-sided IE is associated with better clinical outcomes compared with left-sided IE. This is likely the consequence of multiple factors including right-sided IE patients being younger, there is less systemic embolization and less drug-resistant infection and thus is clinically better-tolerated [5]. Several reports showed that most right-sided IE cases respond to appropriate antibiotic therapy without complications from extra valvular extension, and mortality rates are generally <5% to 10%, even without surgery [5]. Clinical suspicion should always be high for endocarditis in patients with fever and having dialysis catheter. TTE can be negative in many times in these patients; therefore, having a high index of suspicion should always be there in these patients. Non-valvular endocarditis such as superior vena cava endocarditis is a very uncommon presentation and only documented in a few cases in the literature [4].
References
- Rodríguez-Aranda A, Alcazar JM, Sanz F, et Endoluminal colonization as a risk factor for coagulase-negative staphylococcal catheter-related bloodstream infections in haemodialysis patients. Nephrol Dial Transplant. 2011; 26: 948-955.
- Lata C, Girard L, Parkins M, et al. Catheter-related bloodstream infection in end-stage kidney disease: a Canadian narrative review. Can J Kidney Health Dis. 2016; 3: 24.
- Tang S, Beigel R, Arsanjani R, et al. Infective Endovascular Fibrin Sheath Vegetations-A New Cause of Bacteremia Detected by Transesophageal Echocardiogram. Am J Med. 2015; 128: 1029-1038.
- Liang R, Landry I. Endovascular Endocarditis Within the Superior Vena Cava of a Patient with a Tunneled Catheter for Hemodialysis. Cureus. 2022; 14: e23027.
- Shmueli H, Thomas F, Flint N, et al. Right-Sided Infective Endocarditis 2020: Challenges and Updates in Diagnosis and Treatment. J Am Heart Assoc. 2020; 9: e017293.