Enhanced Self Care with Mobile Technology to Reduce Readmission in Congestive Heart Failure: A Pilot Study
Author'(s): Ponrathi Athilingam1*, Miguel A. Labrador2 and Barbara A. Redding3
1Associate Professor, College of Nursing, University of South Florida, United States.
2Professor, College of Computer Science and Engineering, University of South Florida, United States.
3Professor, College of Nursing, University of South Florida, United States.
*Correspondence:
Ponrathi Athilingam, PhD, RN, ACNP, MCH, FHFSA, FAANP, FAAN, Associate Professor, College of Nursing, University of South Florida, United States, Tel: (813) 974-7526.
Received: 28 May 2021; Accepted: 14 June 2021
Citation: Athilingam P, Labrador MA, Redding, BA. Enhanced Self Care with Mobile Technology to Reduce Readmission in Congestive Heart Failure: A Pilot Study. Cardiol Vasc Res. 2021; 5(3): 1-7.
Abstract
Introduction: While people with congestive heart failure (CHF) are living longer, they often have limited ability to engage in treatment plans and self-management at home, thus resulting in increased hospitalizations. The use of novel digital technologies for improving self-management has been recommended for reducing CHF related hospitalizations.
Objective: This pilot study compared a patient-centered enhanced self-care (ESC) intervention using a mobile system called “HeartMapp (HM)” (ESC HM) with an active wait listed control (WLC) group who received CHF education in reducing hospitalization rate and improving CHF outcomes. A total of 18 participants who met the inclusion criteria were block randomized to ESC HM (n=9) and active WLC group (n=9), both using similar mobile platform. Participants were followed for 30-days to examine the number of hospitalization and secondary outcomes including self-care, quality of life, and CHF knowledge.
Results: Results showed that none of the participants randomized to ESC HM (n=9) were hospitalized during the study period of 30-days compared to two participants, both men (11%) in the WLC group. Additionally, clinically meaningful improvement in self-care management (t=3.38; p=0.006) and self-care confidence (6.7 vs 1.8; t=2.53; p=0.028) were observed in ESC HM group. Knowledge significantly improved in the ESC HM group while declined in the WLC group (t=2.37; p=0.037). Quality of life score declined by 2 points in the ESC HM group, while the WLC group score declined by 8 points (p=.08).
Conclusion: Preliminary results of the pilot study demonstrated feasibility and potential efficacy of reducing hospitalization and improving CHF self-care; thus, warranting further evaluation in a well-designed large clinical trial.
Keywords
Introduction
Congestive heart failure (CHF) affects 6.2 million Americans [1] resulting in significant symptom burden and suffering [2]. While people with CHF are living longer, they often have poorer ability to engage in treatment plans, resulting in delayed care seeking for CHF symptoms [3,4], poorer quality of life [5,6] and increased hospitalizations [7,8]. As a result, there is a significant population of CHF patients that are taxing personal, family and healthcare resources [9,10]. For optimal health outcomes, CHF patients are encouraged to follow self-care management strategies at home consisting of complex medications, diet and exercise regimens [11] and modifying behaviors related to CHF symptoms [12]. The complex nature of CHF symptom recognition, lack of patients’ dietary knowledge, and CHF-related physical and psychological distress require interventions that address all of these components, enhance self-care at home to improve quality of life and reduce hospitalization [8,13].
In addition to debilitating CHF-symptoms, age-related physical changes and multiple co-morbidities challenge CHF patients’ ability to learn and continue self-management practices at home [14,15]. Furthermore, many older adults live alone and lack social support often relying on others such as visiting nurses and home care services [16,17], which are often not sustainable in the long- term [18]. Inability to adhere to self-care management negatively impacts hospitalization rates and mortality [19,20]. Nationally, CHF hospitalization rates continue to remain high at 23.2% within 30-days with a median rehospitalization time of 12-days from initial discharge resulting in a mean per-patient cost of $14,631, [1] which is not reimbursed to the hospitals under the Affordable Care Act [21]. Thus, the American Heart Association (AHA) recommends multiple strategies to reduce CHF rehospitalization and the use of novel technologies for improving self-management, thereby reducing rehospitalization [22,23]. To improve self-care and thereby reduce rehospitalization rate, our team designed a patient-centered enhanced self-care (ESC) intervention.
Enhanced Self-Care (ESC) Intervention
The ESC intervention consists of a comprehensive self-management program using a mobile application “HeartMapp (HM)”, (ESC HM). Evidence supports mobile health behavioral interventions to address the rising demand at home for self-management of chronic health conditions [24]. About 95% of Americans own a mobile phone of some kind. One-in-ten American adults are “smartphone-only” internet users and over 50% of mobile phone users are projected to have downloaded at least one health app to their mobile phone [25]. Despite the initial challenges faced by older adults, who often resist using mobile technology, once they join the online world, digital technology often becomes an integral part of their daily lives as it allows them to stay connected with family [26]. Oder adults also tend to offload cognitively demanding processes such as remembering phone numbers and appointments to mobile technology, and thus mobile systems show promise to serve as an external resource, by reducing older adults’ need to use their limited processing capacity to accomplish health care related tasks [27]. Therefore, the ESC HM intervention was developed and refined in our preliminary research [28,29]. The ESC HM system supports self-care and provide feedback to patients on performance.
Design and Method
Our team completed a pilot randomized control trial with block randomization of participants to intervention and control conditions. The primary objective of this pilot study was to compare the feasibility and potential efficacy of the ESC HM intervention to an active wait- listed control (WLC) condition. This paper presents the primary outcome of reduced hospital readmissions at 30-days.
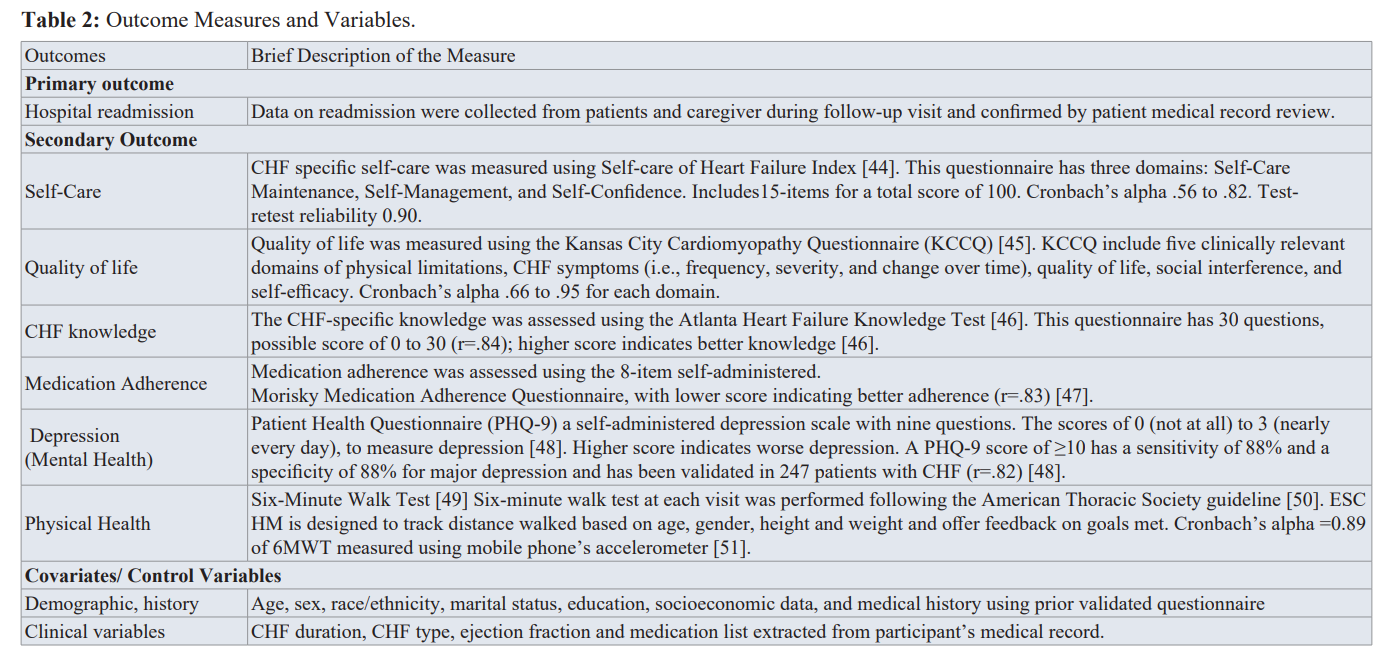
The secondary objective included were improved self-care, quality of life, medication adherence, knowledge, physical and mental health, which were measured using validated questionnaires. The team also collected demographics (i.e., biological sex, age, race, and education), clinical variables (i.e., ejection fraction, and CHF class), and social support. Results of the secondary outcomes at 30-days was published [16]. Table 2 below provide details of the outcome variables measured.
Procedure
The study was approved by the University’s institutional review board (IRB). Participants were recruited from a tertiary hospital, where the IRB-approved brochure was made available at the inpatient units. The discharge care coordinators referred potential CHF participants for the study. Patients were consented and enrolled in the study prior to discharge after a CHF admission or within one-week of discharge from the hospital. If they were discharged to a skilled nursing facility or palliative care, they were deemed not eligible [16].
ESC HM Intervention
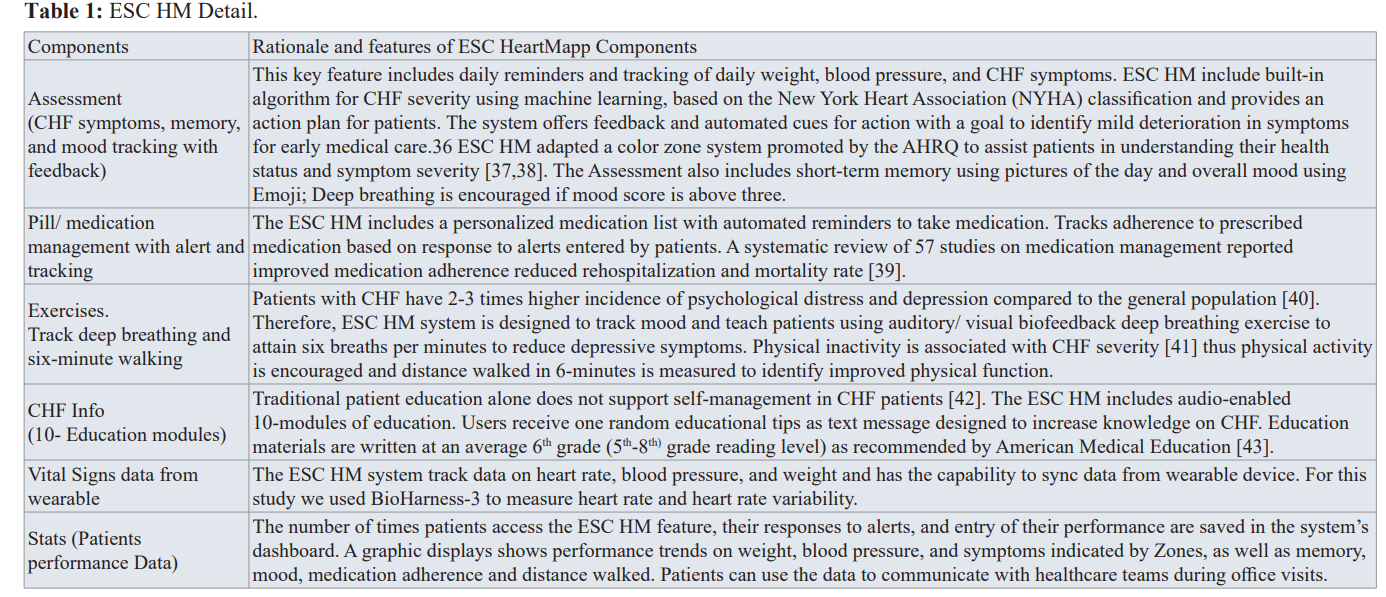
The version of ESC HM tested in this pilot study included 6 key features. The intervention group had ESC HM downloaded onto their own or a loaner Android device. The participants were trained on ESC HM features and given a manual of instruction. Participants received demonstration on responding to tailored reminders to complete daily weighing, CHF symptoms assessment, medication schedule, completing breathing and walking exercise, accessing daily text messages on CHF education and vital sign recording using BioHarness-3. The details of the 6 features of the ESC HM are provided in the Table 1 and Figure 1.
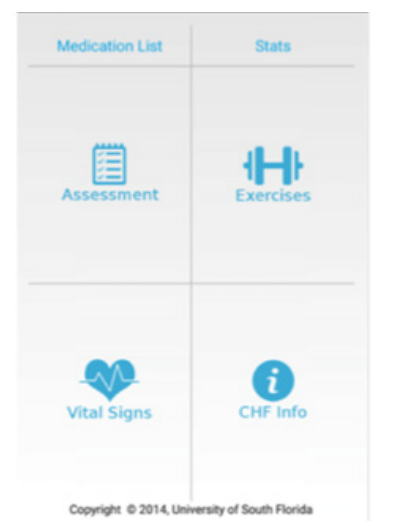
Figure 1: Features of ESC HM.
Active Wait-Listed Control (WLC) Group (n=9)
The WLC control group (n=9) received only CHF education info, one component of the ESC HM downloaded onto their mobile phone. These patients were encouraged to use three modules per week and complete all 10 modules of CHF education by 4 weeks. Participants were assured that they would receive the additional features of the intervention at 4-week follow-up. At the end of the follow-up, they were given access to all five features of ESC HM, except for the vital sign monitoring using BioHarness-3.
Recruitment and Data Collection
The study staff, who have completed HIPPA training, approached the participants who were referred by the discharge care coordinator of the tertiary hospital to inquire whether they are interested in learning more about the study.
The study staff explained the consent process and HIPAA authorization required to allow study staff to access participant health records relevant to the study. Patients who agreed to volunteer and provided consent were screened for eligibility and baseline data obtained before discharge or at the participant’s home within seven-days after discharge from the hospital to complete data collection and training of the interventions. All eligible participants were block randomized to ESC HM or WLC group. Baseline and 30-days follow-up data were collected in an interview format using validated questionnaires. Participant’s medical records were accessed to obtain clinical data on ejection fraction, NYHA Class and medication at baseline.
During follow-up visit at 30-days the conducted at participant’s home, participants were asked about number of emergency room visits or hospital readmissions. This data was confirmed by chart audit. To assure understanding and adherence with treatment training, unblinded study staff called all participants (ESC HM and WLC) three times in the first week after enrollment and thereafter once a week. The study staff was available for questions from all participants to assure training adherence. Participants who did not own an Android device was provided a loaner for use during the study period.
Patient Safety and Monitoring
A study coordinator (doctoral student and nurse practitioner) called all participants 3 times a week during the first week and once a week for the remaining 3 weeks to make sure that they completed their tasks and answered any questions that came up. The study coordinator also checked the ESC HM dashboard daily to monitor participants’ progress and daily statistics including weight and symptoms. The data was used to triage participants early decision making for further management per protocol and if needed referral to their health care providers.
Data Analysis
The statistical software, SAS 9.4 was used for the statistical data analyses in this project [30]. Univariate statistics was conducted on all outcomes, mean standard deviation on continuous variables. Frequency and percentage on categorical variables were performed to describe the sample characteristics of the two groups. Statistical significance was determined using the p value of 0.05. Furthermore, t-tests were performed to compare mean differences between intervention and control groups and between time points (pre and post-intervention scores).
Results
A total of 20 subjects were screened and deemed eligible. One subject gave an incorrect home address and could not be tracked to enroll in the study, and another refused to be a part of the study after eligibility screening. All participants met the inclusion criteria. A total of 18 participants who met the inclusion criteria were block randomized to ESC HM (n=9) and WLC (n=9) to CHF education (CHF info feature of the ESC HM). Only 13 participants (72%) completed the 30-day follow-up with seven from 7 ESC HM group and 6 in the WLC group.
Demographic Data
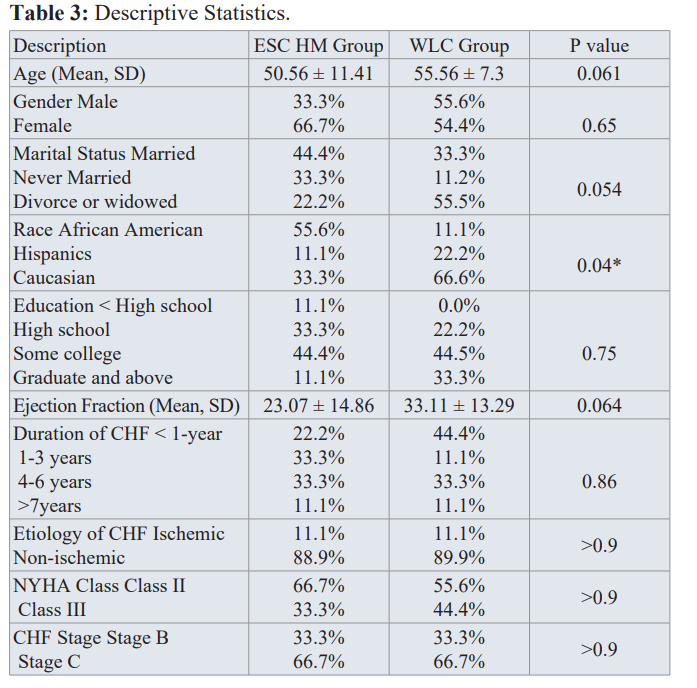
Participants’ in the ESC HM intervention group were younger with a mean age of 50 years with more females (67%); while the WLC group had equal percentage of men and women. About 44% participants in ESC HM group were married; while more participants in WLC were divorced (55%). More African American subjects were enrolled in the ESC HM group (56%) compared to more whites in WLC group (67%). Most subjects (44%) had some college; however, 11.1% had less than high school in the ESC HM group. The intervention group had 10 percent point lower ejection fraction (23%) compared to 33% in the WLC group. The severity of CHF indicated by duration, NYHA class and CHF stage were similar between both groups (See Table 3).
Primary Outcome of Rehospitalization
Hospitalization for CHF was collected from the participants during follow-up interview and were confirmed by chart audit. Results showed that none of the participants who used ESC HM was hospitalized during the study period of 30-days compared to two participants (11%) in the WLC group.
Participants readmitted to hospital in the WLC group were both men aged about 50-years; one was Caucasian and the other Hispanic. Although, this is a pilot study with small sample,results indicated potential efficacy of ESC HM in reducing rehospitalization and warrants a detailed evaluation in a larger diverse sample with long-term follow-up.
Secondary CHF Outcome
Clinically meaningful improvement in all aspects of self- management measured using Self-Care of Heart Failure Index was observed among participants who were randomized to ESC HM compared to WLC group who received a CHF education; [29] with improvement in self-care management (8.7 vs 2.3; t=3.38; p=0.006) and self-care confidence (6.7 vs 1.8; t=2.53; p=0.028). CHF knowledge measured on Atlanta Heart Failure Knowledge Test improved among ESC HM group while knowledge declined in WLC group (3 vs -.66; t=2.37; p=0.037).
Quality of life score on KCCQ declined by 2 points in the WSC HM group, whereas the WLC group declined more by 8 points and was not statistically significant (p=0.08). Depression scores improved in both groups; however, the result was not statistically significant. Medication adherence remained without change in ESC HM group while WLC group declined which was not statistically significant (see Figure 2).
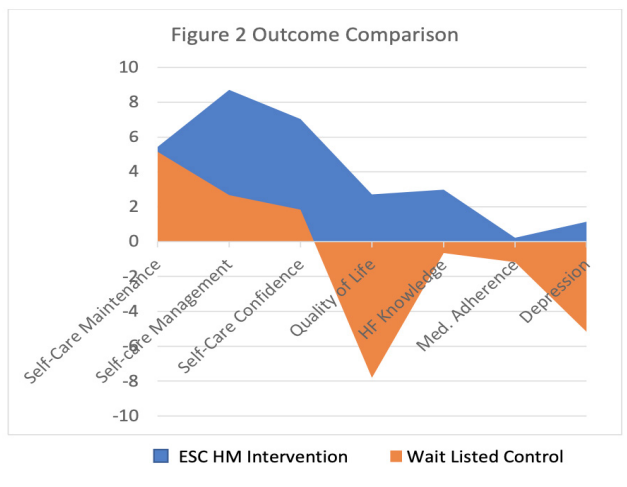
Figure 2: Secondary Outcomes.
Patient Engagement with ESC HM Intervention and Association Outcomes
Participants’ access to ESC HM intervention features were stored and time stamped on a secure website for analysis. Participants accessed symptom assessment, medication tracker and breathing exercise consistently for more than 80% of time. Of all the participants randomized to ESC HM group, four of nine participants (43%) accessed ESC HM daily and completed the assessment of CHF symptoms and exercise (breathing and walking). Five of nine participants (57%) accessed ESC HM features over 24 days (80%) of the time. However, the multivariate regression analysis showed no association between primary and secondary outcomes with patient engagement or time spent by participants accessing ESC HM. Participants reported discomfort and inconvenience of using the chest worn device (BioHarness-3) and thus, accessed vital signs feature for less than 15% of the time.
Participants gave feedback during the follow-up that they prefer a wrist worn device for tracking vital signs and exercise. They also recommended adding a communication feature within the ESC HM system to share and provide access to performance dashboard to health care provider and family members. Participants also recommended adding management of chronic diseases such as hypertension and hyperlipidemia and immunization schedule that have been now added.
Discussion
Given the small sample size, the result is not generalizable and warrants more detailed evaluation in a well-designed clinical trial. The result of this pilot study is promising in that the ESC HM intervention could reduce rehospitalization rates in 30-days and showed trends in improving several CHF outcomes; especially self-care management, self-care confidence, and CHF knowledge. A systematic review on remote telemonitoring interventions reduced the relative risk of all-cause mortality (0.60-0.85) and CHF-related hospitalizations (0.64-0.86) compared with usual care. Improvements in CHF-related hospitalizations appeared to be more pronounced in patients with stable HF in NYHA class II (hazard ratio 0.70; 95% CI 0.34-1.5), indicating appropriate selection of patients who are not in the refractory or end-stage CHF [31]. This is possibly true in this pilot study, as 61% of the total participants, and about 68% in the ESC HM group were in NYHA class II and no participants in CHF stage D and NYHA class IV.
A recent study among adults aged 55 and above in Florida reported that structured telephone support with text messaging improved CHF self-care, knowledge, medication adherence, physical and mental health (P<0.01 for all) [32]. The ESC HM intervention offered daily text messages on educational tips and daily reminders for self-management and medication. Thus, results from this pilot study also supports an earlier study that send text messages to CHF patients that demonstrated significant improvement in CHF self-management [33]. The American Heart Association (AHA) recommends multiple strategies to improve CHF related outcomes and reduce rehospitalizations. Large scale studies are warranted to test novel technologies including text messages and reminders for improving self-management and thereby reducing rehospitalization for CHF [22].
About 80% engagement with ESC HM was noted among all participants randomized to the ESC HM intervention group. This lower engagement was identified because of the challenges faced in using the BioHarness-3 chest strap. Reduced enrollment and attrition rate of about 30% has been reported in a review that is possibly due to several patient factors including participant incentives and time constraint [34]. This is true in that this pilot study was not funded and the participants volunteered for the study that may have caused the dropout and low enrollment. The pilot study faced an attrition rate of 28 % at 30-day follow-up, which is very low. An attrition rate of 35% has been reported in prior studies among CHF population. The reason reported by most participants during follow-up was the use of BioHarness-3 chest Strap. Among participants who completed the follow-up, participants use the chest strap for less than 15% of the time. Participants (80%) gave the team feedback about a potential wrist worn device, One in 5 consumers (21%) in the United States currently uses wearable technology, mostly smart watches or fitness bands [35]. Therefore, the team is currently testing several wrist-worn Bluetooth devices in the laboratory (ie, Microsoft Band-2, Fitbit Charge HR, and Moto 360).
Although, a small sample size, the pilot study enrolled significantly more African American into the ESC HM group (56%). According to a Pew Research Center survey of 2019, African Americans (58%) remain less likely than whites (82%) to own a traditional computer or have high speed internet at home but own smart phone and access internet using smartphone equally (at 80%) [35]. This may have given the study team an advantage to enroll more African Americans.
Conclusion
Lessons learned in this pilot study and the feedback from participants helped the team to refine features in ESC HM to overcome shortcomings for a future larger clinical trial; particularly synchronizing data from wrist worn Bluetooth devices such as Fitbit. In addition, we added a communication link to allow participants to share their dashboard with health care providers and family members. The team also added two additional CHF educational modules to the CHF info feature. The team is also moving towards translating the ESC HM in iOS platform to enhance wide dissemination.
The team propose to secure funding for further exploration of ESC HM in a larger clinical trial to examine short-term and long-term CHF outcomes and reduced rehospitalization rates.
References
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019; 139: 56-66.
- Chamberlain AM, Dunlay SM, Gerber Y, et al. Burden and Timing of Hospitalizations in Heart Failure: A Community Study. Mayo Clin Proc. 2017; 92: 184-192.
- Darling C, Saczynski JS, McManus DD, and et al. Delayed hospital presentation in acute decompensated heart failure: Clinical and patient reported factors. Heart and Lung. 2013; 42: 281-286.
- Ivynian SE, Ferguson C, Newton PJ, et al. Factors influencing care-seeking delay or avoidance of heart failure management: A mixed-methods study. Int J Nurs Stud. 2020; 108: 103603.
- Buck HG, Lee CS, Moser DK, et al. Relationship between self-care and health-related quality of life in older adults with moderate to advanced heart failure. Journal of Cardiovascular Nursing. 2012; 27: 8-15.
- Moradi M, Daneshi F, Behzadmehr R, et al. Quality of life of chronic heart failure patients: a systematic review and meta- analysis. Heart Fail Rev. 2019.
- Sahebi A, Mohammad-Aliha J, Ansari-Ramandi M, et al. Investigation the relationship between self-care and readmission in patients with chronic heart failure. Research in Cardiovascular Medicine. 2015; 4: e25472.
- Madelaire C, Gustafsson F, Kristensen SL, et al. Burden and Causes of Hospital Admissions in Heart Failure During the Last Year of Life. JACC Heart Fail. 2019; 7: 561-570.
- Reilly CM, Butler J, Culler SD, et al. An economic evaluation of a self-care intervention in persons with heart failure and diabetes. J Card Fail. 2015; 21: 730-737.
- Tschope C, Birner C, Bohm M, et al. Heart failure with preserved ejection fraction: current management and future strategies : Expert opinion on the behalf of the Nucleus of the "Heart Failure Working Group" of the German Society of Cardiology (DKG). Clin Res Cardiol. 2018; 107: 1-19.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/ HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017; 136:137-161.
- Granger BB, Ekman I, Hernandez AF, et al. Results of the Chronic Heart Failure Intervention to Improve MEdication Adherence study: A randomized intervention in high-risk patients. Am Heart J. 2015; 169: 539-548.
- Siabani S, Leeder SR, Davidson PM. Barriers and facilitators to self-care in chronic heart failure: a meta-synthesis of qualitative studies. Springerplus. 2013; 2: 320.
- Kamrani AA, Foroughan M, Taraghi Z, et al. Self-care behaviors among elderly with chronic heart failure and related factors. Pak J Biol Sci. 2014; 17: 1161-1169.
- Davies NJ. Improving self-management for patients with long-term conditions. Nursing Standard. 2010; 24: 49-56.
- Sayers SL, Riegel B, Pawlowski S, et al. Social support and self-care of patients with heart failure. Annals of Behavioral Medicine. 2008; 35: 70-79.
- Gallagher R, Luttik ML, Jaarsma T. Social support and self- care in heart failure. Journal of Cardiovascular Nursing. 2011; 26: 439-445.
- Falk H, Ekman I, Anderson R, et al. Older patients' experiences of heart failure-an integrative literature review. Journal of Nursing Scholarship. 2013; 45: 247-255.
- Gupta A, Allen LA, Bhatt DL, et al. Association of the Hospital Readmissions Reduction Program Implementation with Readmission and Mortality Outcomes in Heart Failure. JAMA Cardiol. 2018; 3: 44-53.
- Boyde M, Peters R, New N, et al. Self-care educational intervention to reduce hospitalisations in heart failure: A randomised controlled trial. Eur J Cardiovasc Nurs. 2018; 17: 178-185.
- Bowling B, Newman D, White C, et al. Provider Reimbursement Following the Affordable Care Act. Health Care Manag (Frederick). 2018; 37: 129-135.
- Riegel B, Moser DK, Buck HG, et al. Self-Care for the Prevention and Management of Cardiovascular Disease and Stroke: A Scientific Statement for Healthcare Professionals from the American Heart Association. Journal of the American Heart Association. 2017; 6: 1-27.
- Oyanguren J, Latorre Garcia PM, Torcal Laguna J, et al. Effectiveness and Factors Determining the Success of Management Programs for Patients With Heart Failure: A Systematic Review and Meta-analysis. Rev Esp Cardiol (Engl Ed). 2016; 69: 900-914.
- Lee JA, Choi M, Lee SA, et al. Effective behavioral intervention strategies using mobile health applications for chronic disease management: a systematic review. BMC Med Inform Decis Mak. 2018; 18: 12.
- PewInternet. Mobile Fact sheet in 2019. January 12, 2019 ed. Washington, DC: PewInternet. 2019.
- Seto E, Leonard KJ, Cafazzo JA, et al. Mobile Phone-Based Telemonitoring for Heart Failure Management: A Randomized Controlled Trial". Journal of Medical Internet Research. 2012; 14: 31.
- Morrow D, Chin J. Technology as a Bridge between Health Care Systems and Older Adults. Hershey. PA: IGI Global. 2013.
- Athilingam P, Labrador MA, Remo EFJ, et al. Features and usability assessment of a patient-centered mobile application (HeartMapp) for self-management of heart failure. Applied Nursing Research. 2016; 32: 156-163.
- Athilingam P, Jenkins B, Johansson M, et al. A Mobile Health Intervention to Improve Self-Care in Patients with Heart Failure: Pilot Randomized Control Trial. JMIR Cardio. 2017; 1: e3.
- SAS Instutite. STAT User's Guide Release 8.2. Cary, NC, USA: SAS Institute Inc. 2001.
- Kitsiou S, Pare G, Jaana M. Effects of home tele monitoring interventions on patients with chronic heart failure: an overview of systematic reviews. Journal of Medical Internet Research. 2015; 17: e63.
- Johansson M, Athilingam P. A Dual-Pronged Approach to Improving Heart Failure Outcomes: A Quality Improvement Project. JMIR Aging. 2020; 3: e13513.
- Nundy S, Razi RR, Dick JJ, et al. A text messaging intervention to improve heart failure self-management after hospital discharge in a largely African-American population: before- after study. Journal of Medical Internet Research. 2013; 15: e53.
- Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. 2018; 11: 156-164.
- https://www.pewresearch.org/fact-tank/2020/01/09/about-one-in-five-americans-use-a-smart-watch-or-fitness-tracker/.
- NYHA. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little, Brown & Co. 1994.
- AHRQ. Red-Yellow-Green Congestive Heart Failure (CHF) Tool. In: Quality AfHCRa, ed. Rockville, MD: Department of Health and Human Services. 2007.
- https://www.heartfoundation.org.nz/shop/heart-healthcare/non-stock-resources/heart-failure-action-plan.pdf.
- Ruppar TM, Cooper PS, Mehr DR, et al. Medication Adherence Interventions Improve Heart Failure Mortality and Readmission Rates: Systematic Review and Meta-Analysis of Controlled Trials. J Am Heart Assoc. 2016; 5.
- Rustad JK, Stern TA, Hebert KA, et al. Diagnosis and treatment of depression in patients with congestive heart failure: a review of the literature. Prim Care Companion CNS Disord. 2013; 15.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009; 158: 24-30.
- Cleland JG, Swedberg K, Cohen-Solal A, et al. The Euro Heart Failure Survey of the EUROHEART survey programme. A survey on the quality of care among patients with heart failure in Europe. The Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The Medicines Evaluation Group Centre for Health Economics University of York. European Journal of Heart Failure. 2000; 2: 123-132.
- Athilingam P, Jenkins B, Redding BA. Reading Level and Suitability Assessment of Heart Failure Education in a Mobile App “CHF Info App”. JMIR Aging. 2018.
- Riegel B, Carlson B, Moser DK, et al. Psychometric testing of the self-care of heart failure index. Journal of Cardiac Failure. 2004; 10: 350-360.
- Green CP, Porter CB, Bresnahan DR, et al. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: A new health status measure for heart failure. Journal of the American College of Cardiology. 2000; 35: 1245-1255.
- Reilly CM, Higgins M, Smith A, et al. Development, psychometric testing, and revision of the Atlanta Heart Failure Knowledge Test. Journal of Cardiovascular Nursing. 2009; 24: 500-509.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care Research and Review. 1986; 24: 67-74.
- Pressler SJ, Subramanian U, Perkins SM, et al. Measuring depressive symptoms in heart failure: validity and reliability of the patient health questionnaire-8. American Journal of Critical Care. 2011; 20: 146-152.
- Demers C, McKelvie RS, Negassa A, et al. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. American Heart Journal. 2001; 142: 698-703.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine. 2002; 166: 111-117.
- Brooks GC, Vittinghoff E, Iyer S, et al. Accuracy and Usability of a Self-Administered 6-Minute Walk Test Smartphone Application. Circulation Heart Failure. 2015; 8: 905-913.