Enterobacter Cloacae Device Endocarditis: Case Report, Scoping Study, and Guidelines Review
Author(s): Perry Wengrofsky1, Aron Soleiman1, Fuad Benyaminov1, Filip Oleszak1, Louis Salciccioli2 and Samy I. McFarlane1*
1Department of Internal Medicine, State University of New York, Downstate Medical Center, Brooklyn, N.Y., U.S.A.
2Division of Cardiovascular Disease, Department of Internal Medicine, State University of New York, Downstate Medical Center, Brooklyn, N.Y., U.S.A.
*Correspondence:
Samy I. McFarlane, Distinguished Teaching Professor and Associate Dean, Department of Medicine, Division of Endocrinology, Internal Medicine Residency Program Director, State University of New York-Downstate Medical Center, Brooklyn, New York, Tel: 718-270-3711; Fax: 718-270-6358.
Received: 15 April 2019; Accepted: 23 May 2019
Citation: Perry Wengrofsky, Aron Soleiman, Fuad Benyaminov, et al. Enterobacter Cloacae Device Endocarditis: Case Report, Scoping Study, and Guidelines Review. Cardiol Vasc Res. 2019; 3(3); 1-5.
Abstract
While traditionally an infection of the endocardial surface of heart valves, infective endocarditis (IE), can atypically present as infection of cardiac implantable electronic devices (CIED), including permanent pacemakers (PPM) or automatic implantable cardioverter-defibrillators (AICD). CIED endocarditis, similar to valvular IE, is generally caused by Gram-positive organisms such as Staphylococcus spp., most frequently S. Auerus, but is rarely caused by gram-negative bacteria, both HACEK and non-HACEK species. We present the case of Enterobacter cloacae CIED endocarditis. We also present a scoping study of previous case reports and case series highlighting the risk factors, surgical interventions, and mortality outcomes associated with E. Cloacae endocarditis. We also discuss the current guidelines and recommendations on antibiotic therapies for non-HACEK Gram-negative endocarditis and surgical management of infected CIED extraction and replacement.
Keywords
Introduction
Infective endocarditis (IE), the microbial infection of the endocardium generally affecting cardiac valves, is classified based on diagnostic studies (definite or possible), anatomical involvement (left-sided, involving the mitral and/or aortic valve vs. right-sided, involving the tricuspid and/or pulmonic valve), microbiology (bacterial, fungal), and valve nativity or prostheses [1-3]. Suspected IE is evaluated as definite or possible based on the combination of echocardiographic, microbiologic, laboratory, and physical findings making up the Duke and Modified Duke Criteria [2,4]. Left-sided IE (LSIE) is more common than right-sided IE (RSIE) and affects diverse patient populations with traditional risk factors such as underlying valvular or structural heart disease with or without previous surgery or prosthesis, history of endocarditis, and predisposing clinical characteristics and comorbidities such as advanced age and immunosuppressive populations such as malignancy, cirrhosis, chronic kidney disease, and diabetes mellitus [1,5,6]. The microorganisms most commonly isolated in IE are Staphylococcus species, primarily Staph. aureus, and the HACEK group (Haemophilus spp., Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella spp., Kingella kingae) of Gram-negative rods, and the high rates and associations of these bacteria with IE has resulted in the specific incorporation of these organisms in the micrbiologic parameters of the Duke Criteria [2,7].
In parallel to but significantly rarer than IE affecting cardiac valves is cardiac implantable electronic device (CIED) endocarditis, infection of implanted permanent pacemakers (PPM) or automatic implantable cardioverter-defibrillator (AICD) involving any component of the device, including the intracardiac or large blood vessel region of the electrode lead, the tissue ‘tunnel’ through which the lead travels prior to entering the blood vessel, or the subcutaneous ‘pocket’ device electrical generator [8,9]. CIED endocarditis can result from direct primary infection of any region of the device, most commonly the intracardiac electrode lead, or from secondary seeding in the setting of bacteremia and sepsis of a distant site, and is most frequently caused by Gram-positive organisms, specifically Staph. aureus and Staph.epidermidis, with significantly less cases caused by gram negative rods or Streptococcus species [8-11].
In comparison to the usual causative organisms of bacteremia and both IE and CIED endocarditis, Enterobacter spp., specifically Enterobacter cloacae, is an atypical etiology of bacteremia, and is associated with malignancy, indwelling urinary catheter, and biliary disease [12-14]. While previous case reports and case series on Enterobacter spp. and E. cloacae have presented cases of IE and one instance of PPM endocarditis, surveys of E. cloacae bacteremia point to the urinary tract as the portal for bloodstream seeding, and rarely note endocarditis as the primary source of infection [14-17].
We present, to the best of our knowledge, the first case report of E. cloacae AICD endocarditis. We also present a scoping study of the existing cases and literature on E. cloacae endocarditis to highlight important patient characteristics, clinical findings, and outcomes, and provide a review of antibiotic recommendations for E. cloacae endocarditis and guidelines on device removal and exchange in CIED endocarditis.
Case Presentation
A 57 year-old male with past medical history of heart failure with reduced ejection fraction secondary to non-ischemic cardiomyopathy w/ AICD was directly admitted to the inpatient cardiology service after being called in for positive blood cultures growing E. Cloacae drawn in the emergency department 4 days prior to admission. The patient had initially presented with complaints of fever, malaise, shortness of breath, was tachycardic but hemodynamically stable and without leukocytosis, and was discharged home after blood cultures were drawn.
On admission, the patient was afebrile and hemodynamically stable, and was initially started on zosyn (piperacillin-tazobactam) for suspected endocarditis. Blood work was significant for leukocytosis (12.79K/uL), and elevated C-reactive protein (CRP, 45.8 mg/L), erythrocyte sedimentation rate (ESR, 68 mm/hr), and procalcitonin (.77 ng/mL). He underwent transthoracic echocardiography (TTE) on the day of admission, which revealed ejection fraction of 10% but no vegetations or evidence of endocarditis. Infectious disease was consulted, and recommended transitioning to cefepime and urgent removal and replacement of the AICD. On hospital day 3, the patient underwent transesophageal echocardiography (TEE) which revealed right atrial (RA) dilation, several small mobile masses in the RA cavity associated with the device wire consistent with small vegetations, and a larger “matted-appearing” region associated with the device wire consistent with vegetation (Image 1).
On hospital day 9, the patient underwent unsuccessful percutaneous AICD lead extraction, with remaining leads showing evidence of infection requiring complete removal. The patient was tachycardic and hypotensive post procedure, and was transferred to the coronary care unit where he was started on milrinone and norepinephrine for cardiogenic shock. Cardiothoracic surgery was consulted for open chest surgery for lead removal. By hospital day 14, the patient was successfully titrated off vasopressor and inotropic support. On hospital day 15, the patient underwent sternotomy with lead extraction and device reimplantation, and he was transferred postoperatively to the cardiothoracic intensive care unit on ventilator and vasopressor support.
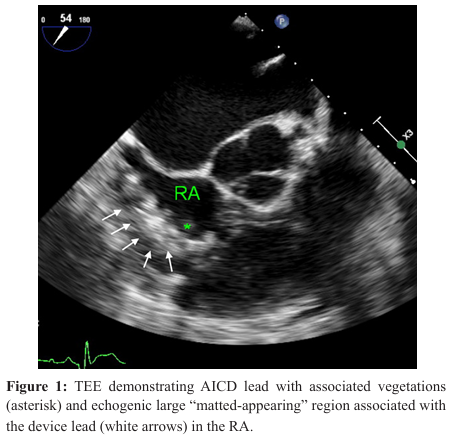
On postoperative day 1 (hospital day 16), the patient was extubated, and was titrated off of vasopressor and inotropic support by postoperative day 5 (hospital day 20). He remained in the cardiothoracic intensive care unit where he had peripherally inserted central catheter (PICC) placement for continued antibiotic course and participated in physical therapy regimen. On hospital day 30 (postoperative day 15), the patient became hypotensive and was started on dobutamine for cardiogenic shock. A few hours later, the patient became unresponsiveness and pulseless, and cardiopulmonary resuscitation was initiated. The sternotomy was opened at bedside and manual cardiac massage was performed with direct intramyocardial and aortic root injection of epinephrine. Return of spontaneous circulation was achieved, and the patient was transferred emergently to the operation room for surgical wash out, and placement of Swan-Ganz catheter and temporary chest closure. Upon arrival in the operating room, the patient hemodynamically decompensated, manual cardiac massage was restarted, direct intramyocardial and aortic root epinephrine was injected, and bipolar epicardial pacing leads were also placed. Resuscitation efforts were unsuccessful and the patient expired.
Scoping Study
Materials and Methods
On December 31, 2018, a systematic search for a scoping study was conducted using Pubmed using keywords “infective endocarditis, enterobacter, enterobacter cloacae, cardiac device endocarditis” to identify individual case reports and cases series of Enterobacter spp. and E. Cloacae endocarditis. Case reports were reviewed individually, and when the full text in hard print copies in journal stacks, clinical data and information was interpreted from case series referencing the specific case report. All case reports and case series were reviewed and analyzed for demographic data including age and gender, past medical history, echocardiographic (either transthoracic or transesophageal echocardiography) findings, sites of septic embolism, antibiotic and surgical management, whether E. Cloacae was only species isolated or if there was evidence of polymicrobial infection and bacteremia, and clinical outcomes (survival or death).
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) individual case reports, case series, or case reports with accompanying reviews. (2) The patients in the cases were adults older than 18 years of age. (3) Study results could be either quantitative representation of the rates or frequency of specified clinical characteristics, or a qualitative representation of specified clinical characteristics. Exclusion criteria were as follows: (1) non-English language articles or English language articles without an available full text version; (2) articles that contained data that significantly overlapped with that of another published report.
Results
Systematic search revealed 13 cases of E. cloacae endocarditis meeting inclusion criteria [18-30], resulting in 14 total cases (including the present case) for scoping study (Table 1). The mean age was 50.2 years old with a standard deviation of 15.7. 28.6% of patients had underlying rheumatic heart disease or valvular disease, with or without previous replacement. 35.8% of patients had immunocompromising conditions such as diabetes mellitus,
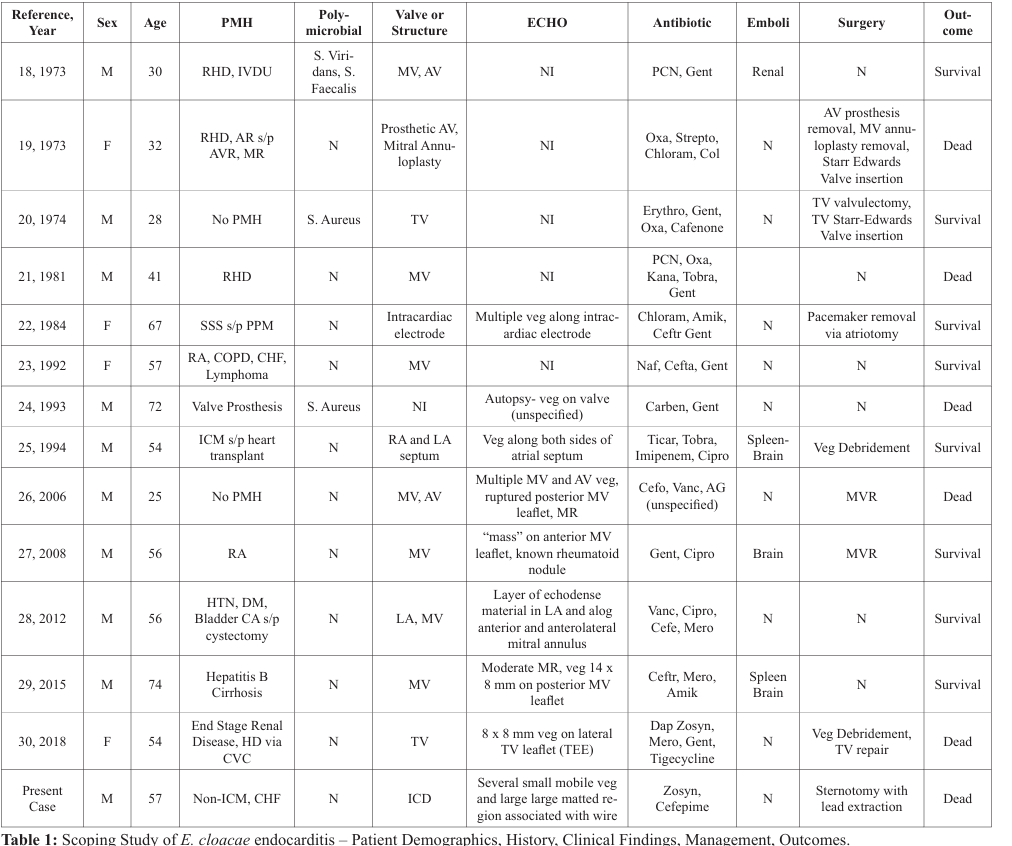
Key: M: male, F: Female, PMH: Past Medical History, RHD: Rheumatic Heart Disease, IVDU: Intravenous Drug Use, AR: Aortic Regurgitation, s/p: Status Post, AVR: Aortic Valve Replacement, MR: Mitral Regurgitation, SSS: Sick Sinus Syndrome, RA: Rheumatoid Arthritis, COPD: Chronic Obstructive Pulmonary Disease, CHF: Congestive Heart Failure, ICM: Ischemic Cardiomyopathy, HTN: Hypertension, DM: Diabetes Mellitus, CA: Cancer, HD: Hemodialysis, CVC: Central Venous Catheter, MV: Mitral Valve, AV: Aortic Valve, RA: Right Atrium, LA: Left Atrium, TV: Tricuspid Valve, Veg: Vegetation, PCN: Pencillin, Gent: Gentamicin, Oxa: Oxacillin, Strepto: Streptomycin, Chloram: Chloramphenicol, Col: Colistin, Erythro: Erythromycin, Kana: Kanamycin, Tobra: Tobramycin, Amik: Amikacin, Ceftr: Ceftriaxone, Naf: Nafacillin, Ticar, Cipro: Ciprofloxacin, Cefo: Cefotaxime, Vanc: Vancomycin, Cefe: Cefepmime, Mero: Meropenem, Dap: Daptomycin, N: no, S. Viridans: Streptococcus Viridans, S. Faecalis:
Streptococcus faecalis.
cirrhosis, rheumatoid arthritis, or malignancy. 57.1% of patients had mitral valve (native or prosthesis) involvement, and 21.4% of patients have multiple valve (or paravavular region such as mitral annulus) involvement. 21.4% of patients had polymicrobial infection. Surgery was performed in 42.9% of patients, and the overall mortality rate of E. Cloacae endocarditis was 42.9%.
Discussion
While E. Cloacae endocarditis has been documented in previous case reports and case series, with only one case of CIED endocarditis of a PPM, E. Cloacae endocarditis remains very uncommon, with this case representing the first reported case of E. Cloacae ICD endocarditis. This patient, a case of E. Cloacae PPM endocarditis, and a case of Enterobacter aerogenes represent the only cases of Enterobacter spp. CIED endocarditis [22,31]. The predominance of mitral valve involvement in E. Cloacae endocarditis is consistent with general patterns of valvular involvement in Enterobacter spp. endocarditis as reported by Moon et al., as well as the propensity for pre-existing rheumatic heart disease, valvular stenosis or insufficiency, and prosthesis [28]. The mortality rate of 42.9% for E. Cloacae endocarditis is similar to the mortality rate for 44.4% mortality rate for reported cases of E. Aerogenes endocarditis [28,31]. While the clinical entity of E. Cloacae endocarditis is rare, even more so presenting as CIED endocarditis, the high mortality rate associated requires prompt diagnostic evaluation, antibiotic management, and when indicated, surgical intervention when a patient presents with E. Cloacae and suspected IE.
The highly uncommon nature of E. Cloacae IE, Enterobacter spp. IE, and IE caused by other Gram-negative bacilli (nonHACEK) has led current guidelines on management to consider surgical management as a reasonable plan of care alongside a prolonged course of antibiotics. According to the guidelines endorsed by the Infectious Disease Society of America, cardiac surgery is considered reasonable in combination with prolonged course of antibiotics, with antibiotic regimens including six weeks of combination beta-lactams with either an aminoglycoside or fluoroquinolone considered reasonable [2]. Despite the high rates of cardiac surgery in cases of non-HACEK Gram-negative endocarditis, the mortality rate remains very high, with inhospital mortality rates considerably higher than in HACEK endocarditis [32,33]. Current guidelines and recommendations endorsed by the Heart Rhythm Society recommend that in cases of infected CIED, after completion of TEE for evaluation of vegetation presence, size, character, and potential embolic risk, lead extraction is recommended with appropriate consultations between electrophysiologists, interventional cardiologists, and cardiac surgeons for surgical planning [34]. CIED reimplantation is recommended after blood cultures are negative for at least 72 hours or longer depending on the specified clinical picture [34].
Our patient’s hemodynamic decompensations and progression to cardiogenic shock, both after unsuccessful percutaneous lead extraction, and sternotomy with open surgical lead extraction and device reimplantation, in the context of extensive large vegetations demonstrate the difficulty and possible cardiovascular compromise that can result from surgical intervention. As seen in our patients, the risk factors for larger vegetations in CIED endocarditis include low ejection fraction and AICD, with vegetations developing more rapidly in patients with aforementioned risk [35]. Mortality in CIED endocarditis is high, but overall extraction-related rates are less than 1%, with extraction complications more frequently associated with open-heart surgical device removal [36].
In summary, we present the first case report of E. Cloacae ICD endocarditis. Scoping study of previous cases of E. Cloacae endocarditis highlights the rarity and highly unusual nature of associated CEID endocarditis. While guidelines and recommendations exist for the evaluation of CIED endocarditis in HACEK and non-HACEK Gram-negative species and
device extraction, further work is needed to understand speciesspecific strategies for diagnostic workup and management with echocardiographic testing and timing of surgical intervention in patient’s with suspected E. Cloacae endocarditis, both CIED and valvular IE.
Acknowledgement
This work is supported, in part, by the efforts of Dr. Moro O. Salifu M.D., M.P.H., M.B.A., M.A.C.P., Professor and Chairman of Medicine through NIH Grant number S21MD012474.
References
- Cahill TJ, Prendergast BD. Infective Endocarditis. Lancet. 2016; 287: 882-893.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Endorsed by: Infectious Disease Society of America. Circulation. 2015; 132: 1435-1486.
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. JACC. 2017; 69: 325-344.
- Buchi A, Hoffmann M, Zbinden S, et al. The Duke Minor Criterion “Predisposing Heart Condition” in Native Valve Infective Endocarditis – a Systematic Review. Swiss Med Wkly. 2018; 148: w14675.
- Werdan K, Dietz S, Loffler B, et al. Mechanisms of Infective Endocarditis: Pathogen-Host Interaction and Risk States. Nat Rev Cardiol. 2014; 11: 35-50.
- Klein M, Wang A. Infective Endocarditis. J Intensive Care Med. 2014; 31: 151-163.
- Pant S, Patel NJ, Deshmukh A, et al. Trends in Infective Endocarditis Incidence, Microbiology, and Valve Replacement in the United States From 2000 to 2011. JACC. 2015; 65: 2070-2076.
- Edelstein S, Yahalom M. Cardiac Device-related Endocarditis: Epidemiology, Pathogenesis, Diagnosis and Treatment–a Review. Int J Angiol. 2009; 18: 167-172.
- Baddour LM, Epstein AE, Eerickson CC, et al. Update on Cardiovascular Implantable Electronic Device Infections and their Management: a Scientific Statement from the American Heart Association. Circulation. 2010; 121: 458-477.
- Maskarinec SA, Thaden JT, Cyr DD, et al. The Risk of Cardiac Device-related Infection in Bacteremic Patients is Species Specific: Results of a 12-year Prospective Cohort. Open Forum Infect Dis. 2017; 4: 132.
- Polewczyk A, Jachec W, Polewcyzk AM, et al. Infectious Complications in Patients with Cardiac Implantable Electronic Devices: Risk Factors, Prevention, and Prognosis. Pol Arch Intern Med. 2017; 127: 597-567.
- Kang C, Chung DR, Ko KS, et al. Clinical Predictors of Enterobacter Bacteremia among Patients Admitted to the ED. Am J Emerg Med. 2012; 30: 165-169.
- Harris PNA, Peri AM, Pelecanos AM, et al. Risk Factors for Relapse or Persistence of Bacteraemia Caused by Enterobacter spp.: a Case-Control Study. Antimicrob Resist Infect Control. 2017; 6: 14.
- Juanjuan D, Zhiyong Z, Xiaoju L, et al. Retrospective Analysis of Bacteremia because of Enterobacter cloacae Compared with Escherichia coli Bacteremia. Int J Clin Pract. 2007; 61: 583-588.
- Song EH, Park KH, Jang EY, et al. Comparison of the Clinical and Microbiologic Characteristics of Patients with Enterobacter cloacae and Enterobacter aerogenes Bacteremia: a Prospective Observation Study. Diagn Microbiol Infect Dis. 2010; 66: 436-440.
- Moon J, Smith T, Sahud AG, et al. An Unusual Etiology of Infective Endocarditis: Enterobacter cloacae. J Infect Chemother. 2012; 18: 925-930.
- Daelemans R, Kersschot I, Van den Branden F, et al. Pacemaker Endocarditis: Contribution of Two-dimensional Echocardiography. Acta Cardiol. 1984; 39: 293-299.
- Menda KB, Gorbach SL. Favorable Experience with Bacterial Endocarditis in Heroin Addicts. Ann Intern Med. 1973; 78: 2532.
- Iannini PB, Hull SF, Quintillani R. Severe Sepsis from Enterobacter. Arch Surg. 1973; 107: 854-865.
- Simberkoff MS, Isom W, Smithivas T, et al. Two-stage Tricuspid Valve Replacement for Mixed Bacterial Endocarditis. ArchIntern Med. 1974; 133: 212-216
- Bortolotti U, Thiene G, Milano A, et al. Pathological Study of Infective Endocarditis on Hancock Porcine Bioprosthesis. J Thorac Cardiovasc Surg. 1981; 81: 934-942.
- Daelemans R, Kersschot I, Van der Branden F, et al. Pacemaker Endocarditis: Contribution of Two-dimensional Echocardiography. Acta Cardiol. 1984; 39: 293-299.
- Tunkel AR, Fisch MJ, Schlein A, et al. Enterobacter Endocarditis. Scand J Infect Dis. 1992; 24: 233-240.
- Fang G, Keys TF, Gentry LO, et al. Prosthetic Valve Endocarditis Resulting from Nosocomial Bacteremia. A Prospective, Multicenter Study. Ann Intern Med. 1993; 119: 560-567.
- Toporoff B, Rosado LJ, Appleton CP, et al. Successful Treatment of Early Infective Endocarditis and Mediastinitis in a Heart Transplant Recipient. J Heart Lung Transplant. 1994; 13: 546-548.
- Aubron C, Charpentier J, Trouillet JL, et al. Native-valve Infective Endocarditis Caused by Enterobacteriaceae: Report on 9 Cases and Literature Review. Scand J Infect Dis. 2006; 38: 873-881.
- Giladi H, Sukenik S, Flusser D, et al. A Rare Cause of Enterobacter Endocarditis Superimposed on a Mitral Valve Rheumatoid Nodule. J Clin Rheumatol. 2008; 14: 97-100.
- Moon J, Smith T, Sahud AG, et al. AnUsual Etiology of Infective Endocarditis: Enterobacter Cloacae. J Infect Chemother. 2012; 18: 925-930.
- Yoshino Y, Okugawa S, Kimura S, et al. Infective Endocarditis due to Enterobacter Cloacae Resistant to Third- and Fourthgeneration Cephalosporins. J Microbiol Immunol Infect. 2015; 48: 226-228.
- Karashian O, Yildiz Z, Unal O, et al. A Rare Cause of Healthcare-associated Infective Endocarditis: Enterobacter cloacae. IDCases. 2018; 12: 18-20.
- Dickinson G, Rodriguez K, Arcey S, et al. Efficacy of Imipenem/cilastatin in Endocarditis. Am J Med. 1985; 78: 117-121.
- Morpeth S, Murdoch D, Carbell CH, et al. Non-HACEK Gram-negative Bacillus Endocarditis. Ann Intern Med. 2007; 147: 829-835.
- Chambers ST, Murdoch D, Morris A, et al. HACEK Infective Endocarditis: Characteristics and Outcomes from a Large, Multi-National Cohort. PLoS One. 2013; 8: e63181.
- Kusumoto FM, Schoenfeld MH, Wilkoff, BL, et al. 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction. Heart Rhythm. 2017; 14: 503-551.
- Poleczyk A, Jachec W, Tomaszewski A, et al. Lead-related Infective Endocarditis: Factors Influencing the Formation of Large Vegetations. Europace. 2017; 19: 1022-1030.
- Kirkfeldt RE, Johnson JB, Nielsen JC. Management of Cardiac Electronic Device Infections: Challenges and Outcomes.Arrhythm Electrophysiol Rev. 2016; 5: 183-187.