Epidemiological, Clinical Aspects, Risk Factors and Paraclinques and Therapeutics of Chronic Viral Hepatitis C at the Donka National Hospital Chu Conakry
Author'(s): Diallo Mamadou Sarifou1,2*, Youssouf Oumarou3, Wann Thierno Amadou5,2, Pare Stella Line Emmanuella7, Diallo Ahmed Tidiane1,2, Diallo Kadiatou1,2, Diallo Djenabou1,2, Bah Mamadou Lamine Yaya5,2, Diakhaby Mamadou5, Kanté Mamadou Aliou5, Sylla Djibril5,2, Soro Dramane4, Diallo Abdourahmane N'Djouria6
1Hepato-gastroenterology department of the Donka national hospital, Conakry, Guinea.
2Faculty of Health Sciences and Techniques, Gamal Abdel Nasser University, Conakry, Guinea.
3Bangui Community University Hospital Center, Internal Medicine Department, Central African Republic.
4Hepato Gastroenterology Department, Cocody University Hospital, Abidjan, Ivory Coast.
5Department of Internal Medicine, Donka National Hospital, Conakry, Guinea.
6SOS Hepatitis Guinea.
7Hepato-Gastroenterology Department of the Regional Hospital Center of Fada N'Gourma, Burkina Faso.
*Correspondence:
Dr. Mamadou Sarifou DIALLO, Hepato-Gastroenterology Department Donka National Hospital CHU Conakry, Guinea, Tel: 00224628690551.
Received: 04 May 2024 Accepted: 24 Jun 2024
Citation: Diallo Mamadou Sarifou, Youssouf Oumarou, Wann Thierno Amadou, et al. Epidemiological, Clinical Aspects, Risk Factors and Paraclinques and Therapeutics of Chronic Viral Hepatitis C at the Donka National Hospital Chu Conakry. Gastroint Hepatol Dig Dis. 2024; 7(3): 1-7.
Abstract
Introduction: Viral hepatitis C is a real public health problem worldwide due to its complications, notably cirrhosis and hepatocellular carcinoma.
Patients and Method: This was a descriptive cross-sectional study including all patients followed in our outpatient department in the period from January 1, 2022 to March 30, 2024, i.e. 27 months. This work aimed to study the epidemiological, clinical and paraclinical aspects of patients carrying HCV and followed in our department.
Results: Of the 2242 patients followed, 83 patients had positive anti-HCV antibodies, i.e. a prevalence of 3.70%; 46 were men (55.7%) and 37 women (44.3%), for a sex ratio of 1.25. The average age was 46 years with a range of 22 to 80 years. The main risk factors were: dental care 43.37%, surgery 24.09%, blood transfusion and/or its derivatives 12.04%, circumcision and/or excision 100%. The main circumstances of discovery of viral hepatitis C were: physical asthenia (43.37%), cytolysis and jaundice in 36.14%, during voluntary screening in 14.46% and donation of blood in 6.02% of cases; 56% of our patients had a viral load (HCV RNA) less than 50,000 IU/ml and 44% had a viremia greater than 50,000 IU/ml. Abdominal ultrasound was normal in 80% of cases, hepatic steatosis in 10% of cases. The assessment of fibrosis by Fibroscan showed absence of fibrosis or minimal fibrosis (F0F1) in 36.14% (n=30), moderate fibrosis (F2) in 30.12% (n=25); severe fibrosis (F3) in 21.69% (n=18) and cirrhosis (F4) in 12.05% (n=10).
Conclusion: The prevalence of HCV infection is very high in this study, the treatment borne by patients in our poor countries remains too expensive, only prevention remains an option. Therapeutically, all our patients (100%) were placed on SOFOSBUVIR 400mg and VELPATASVIR 100mg (SOFOVELPA). Ribavirin added in four of our cirrhotic patients. Sustained virological response (SVR) was achieved in 80 of our patients. HCV RNA was undetectable in all 77 patients after twelve weeks of treatment with SOFOVELPA, undetectable in 3 patients after 24 weeks of treatment with SOFOVELPA + RIBAVIRIN. The three HCC patients died during treatment in the third month; fourth month and eighth month. SVR was 100% in patients who completed their treatment.
Keywords
Introduction
Hepatitis C virus (HCV) infection today represents one of the most frequent causes of chronic liver disease and hepatocellular carcinoma (HCC) [1]. It is an endemic pathology in sub-Saharan Africa [2]. Acute infection can heal or become chronic. Chronic forms can evolve insidiously over years and become complicated. Indeed, the chronicity of liver damage leads to a process of fibrosis resulting from a proliferation and accumulation of myofibroblastic cells following the inflammatory reaction. This is a process common to the various etiologies of chronic liver disease and the evolution of this fibrosis is a determining factor in the occurrence of complications such as cirrhosis and hepatocellular carcinoma which are life-threatening [3]. Transmission occurs mainly through percutaneous or mucosal exposure to blood, particularly during injections or medical procedures, through sexual contact and through venous or intranasal drug use. The means of prevention of viral hepatitis C are transfusion safety, aseptic injections and ear piercings and avoiding practices leading to skin breakdown (scarification, tattooing, acupuncture, etc.) [3-5]. In Guinea, data relating to viral hepatitis C in our department are not available. It is in view of this state of affairs that we carried out this work, the aim of which was to study the epidemiological, clinical and paraclinical aspects of patients carrying HCV and followed in our department.
Material and Methods
We carried out a descriptive cross-sectional study covering all patients followed in our department during the study period. It was carried out from January 1, 2022 to March 30, 2024, i.e. 27 months in outpatient consultation of the hepato-gastroenterology department of the Donka CHU national hospital in Conakry. Included were patients of all ages, both sexes, from all origins followed in outpatient consultation in the department during the study period and who agreed to participate in the study by oral consent in whom anti-HCV antibodies are positive with a detectable viral load (viremic patients).
The criteria for non-inclusion of patients were:
− Patients with positive anti-HCV antibodies with undetectable viremia
− Patients with positive anti-HCV antibodies who did not have a viral load
− Patients with positive anti-HCV antibodies who refused to participate in the study
− Patients with positive anti-HCV antibodies and a positive viral load with limited financial means (patients who were unable to purchase their medication)
Sociodemographic parameters
− Age
− Sex
Clinical parameters
− Reason for consultation
− The patient's personal history: medical, surgical, risk factors for contamination: blood transfusion, tattooing, scarification, dental care, hemodialysis, intravenous drug use.
− Clinical data collected at admission were the vital constants
Biological parameters
− Transaminases, particularly ALT
− Serum creatinine for pre-therapeutic assessment
− Viral markers: HBV, HCV, HDV, retroviral serology (SRV), ELISA technique was used
− For the HCV RNA viral load, quantification was carried out by real-time PCR (RT PCR real-time Gene Xpert) with a quantification range: 10 – 1,000,000,000 IU/ml or 1-8.8 Log. The patients were divided into two groups: those with a viral load less than 50,000 IU/mL and those with a viremia greater than 50,000 IU/mL.
− The criteria for starting treatment were anti-HCV antibodies are positive with a detectable viral load.
Radiological parameters
Abdominal ultrasound or abdominal scanner: to assess the intra- abdominal organs; especially look for hepatic dysmorphia, signs of portal hypertension (PH), a nodule suspicious for hepatocellular carcinoma (HCC) and/or the kinetics of HCC “Wash out”; appreciate the abundance of ascites
Fibroscan: The classification of liver fibrosis was made using the Fibroscan result expressed in kPa and the fibrosis results are classified into the following stages:
− Absence of fibrosis or minimal fibrosis = F0 F1, when liver elasticity is less than 7.5 kPa
− Presence of F2 fibrosis when liver elasticity is between 7 and 9.5 kPa
− Presence of severe fibrosis F3, when liver elasticity is between 9.5 and 13 kPa
− Presence of F4 cirrhosis, when liver elasticity is greater than 13 kPa
The treatment regimen was SOFOSBUVIR 400mg and VELPATASVIR 100mg (SOFOVELPA) in combination for 12 weeks for patients with positive HCV with detectable RNA and those with compensated cirrhosis and SOFOSBUVIR 400mg and VELPATASVIR 100mg plus RIBAVIRIN 200mg according to the patient's weight for the patients with decompensated cirrhosis for 24 weeks; i.e. 1000mg of ribavirin for a weight less than 75kg and 1200mg for a weight greater than 75kg, dose administered twice a day.
Genotyping was not carried out for financial reasons justifying the use of pan genotypic molecules in all our patients. Sustained viral response (SVR) is defined by undetectable HCV RNA at 12 and 24 weeks after the end of treatment.
Data collection, entry and analysis:
− Data collection was carried out using a survey form established for this purpose (see appendices)
− Data entry was done in EPI-INFO VERSION 7
− The tests used for data analysis were the Pearson Chi-square test.
− The alpha threshold was set at 5%
Results
Of the 2242 patients followed in our department during the study period, 83 patients had positive anti-HCV antibodies, representing a prevalence of 3.70%; 46 were men (55.7%) and 37 women (44.3%), i.e. a M/F sex ratio of 1.25.
The average age of our patients was 46 years with a range of 22 to 80 years. The 30-50 year old age group was the most affected with a frequency of 56%.
The history and main risk factors for contamination of our patients are presented in the table below (Table 1).
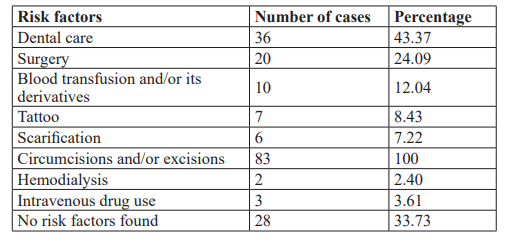
The circumstances of discovery of viral hepatitis C were: physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14 .46% (n=12) and blood donation in 6.02% (n=5).
On the virological level, 56% of our patients had a viral load (HCV RNA) less than 50,000 IU/mL and 44% had a viremia greater than 50,000 IU/mL. Three of our patients had hepatitis B surface antigen and two patients had HCV and HIV co-infection.
According to biochemical parameters, ALT transaminases were shown in the figure below (Figure1).
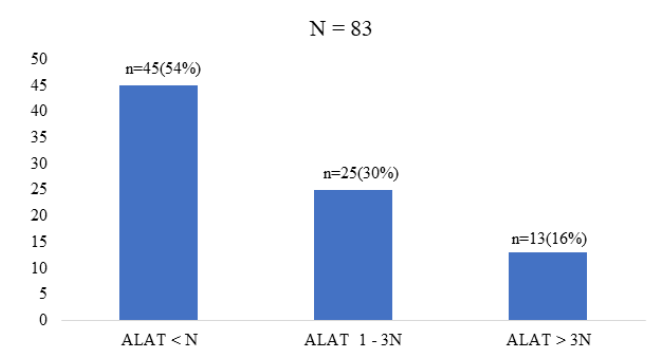
Figure 1: Biochemical parameters.
Abdominal ultrasound was normal in 80% of cases, hepatic steatosis in 10% of cases, biliary cysts in two patients, cirrhosis in six cases with nodules suspicious for HCC in three patients. Portal trunk dilatation in two patients. CT scan found the kinetics of HCC with “Wash out” in these three patients. The evaluation of liver fibrosis was carried out by Fibroscan. Impulse elastometry showed absence of fibrosis or minimal fibrosis (F0F1) in 36.14% (n=30), moderate fibrosis (F2) in 30.12% (n=25); severe fibrosis (F3) in 21.69% (n=18) and cirrhosis (F4) in 12.05% (n=10).
Therapeutically, all our patients (100%) were placed on SOFOSBUVIR 400mg and VELPATASVIR 100mg (SOFOVELPA). Ribavirin added in four of our cirrhotic patients. Sustained virological response (SVR) was achieved in 80 of our patients. HCV RNA was undetectable in all 77 patients after twelve weeks of treatment with SOFOVELPA, undetectable in 3 patients after 24 weeks of treatment with SOFOVELPA + RIBAVIRIN. The three HCC patients died during treatment in the third month; fourth month and eighth month. SVR was 100% in patients who completed their treatment.
Discussion
The small size of our sample, the cost of certain tests, particularly the viral load of hepatitis C, the unavailability of this test at the local level, the high cost of treatment which is entirely the responsibility of the patient, the monocentric nature of the study were, among other things, the main limitations of this study. But, nevertheless, this study made it possible to determine the prevalence and identify the risk factors for contamination of viral hepatitis C in our department and to give an idea of the results of treatment with direct-acting antivirals in this case the association SOFOSBUVIR 400mg and VELPATASVIR 100mg (SOFOVELPA). We collected 83 cases out of 2242 patients followed in our department during the study period, i.e. a prevalence of 3.70%. This prevalence is superimposable to that of TOURE M et al. [6] who, during an evaluation of the overall prevalence of hepatitis C virus antibodies in 2005 among 400 patients hospitalized at the Conakry University Hospital, 2.7% of Reactive sera (11/400) were confirmed positive and close to that of KPOUSSOU AR et al. [7] in Benin who reported a hepatitis C seroprevalence of 5.7% in their study. Our result is in line with international estimates. Thus, it is estimated that approximately 3% of the world population, or 170 million individuals, are infected with HCV. In Europe, there is a north-south gradient in the prevalence of anti-HCV antibodies ranging from 0.5% in northern countries to around 2% in countries around the Mediterranean [8]. We noted a male predominance of 55.7% with a sex ratio of 1.25. This result is identical to that of de KPOUSSOU AR et al. [7] in Benin who found a male predominance of 65.5% with a sex ratio of 1.85. As for DIALLO MS et al. [9] out of a total of 200 patients included in their study, 103 were men (51.5%) and 97 women (48.5%), i.e. a M/F sex ratio of 1 .06. However, a male predominance has been reported in other studies with percentages up to 67% [10]. This male predominance could be explained by the lifestyle of men exposing themselves to contact with risk factors more often than women [9]. The average age of our patients was 46 years with a range of 22 years and 80 years old. KPOUSSOU AR et al. [7] in Benin reported a mean age of 39.7 years ± 13.5 years for patients carrying HBV and 59.3 years ± 14.3 years for HCV carriers. In Burkina Fasso, [11] the average age of patients was 47 ± 10 years with extremes of 23 and 71 years. The study of risk factors for HCV transmission in our patients showed circumcision and/or excisions (100%), a predominance of dental care (43.37%) (Table 1). The practice of circumcision and/or excision is linked to traditional and religious considerations; these were done using the same instruments and carried out during a group of a large number of people and with a lack of sterilization. COUZIGOU P et al., mentioned a predominance of dental care in 68.4% [12], this mode of contamination is rarely incriminated in certain countries such as Tunisia (0.63%) [13]. History of surgery was noted in 24.09% of cases. These antecedents may be susceptible to a source of contamination and this could be explained by the fact of the use of reusable (non-disposable) materials and the fact that the instrument sterilization measures were not sufficiently effective to eliminate the HCV. The causal link with the surgical procedure cannot be established with certainty. In a Tunisian study, the history of surgical intervention was noted in 20% of cases [13]. The transfusion of blood and its derivatives found in 12.04% in our series. At the international level, the transfusion of blood products and derivatives played a major role in the spread of HCV infection until 1990. According to a study carried out by a French team in 2005, the suspected risk factors were: transfusion was responsible for chronic HCV infection in France 38.8%., intravenous drug use (21%), nasal drug use (6.2%), occupational exposure (5, 5%), nosocomial exposure (22.6%) and no risk factors found in 14.7% [14]. Likewise, DIALLO MS et al. [9] noted blood transfusion in 4.5% of cases and dental care in 4% of cases as the main risk factors. In Benin KPOUSSOU AR et al. [7] found scarification as the predominant risk factor with a frequency of 55.1%. As was demonstrated in our study, tattoos (8.43) and scarifications (7.22%) played a significant role in the transmission of HCV. Finally, no risk factors were found in 33.73% of our patients (Table 1). For other authors, the mode of contamination could not be identified in 8 to 20% of cases [13]. In these patients, either there is an as yet unknown mode of contamination or it is more likely an unknown percutaneous transmission: mass vaccination, scarification, circumcision, piercing, sharing of personal hygiene objects, intra- family, injection of medication using non-disposable equipment. These situations are present in our country with limited resources. The circumstances of discovery of viral hepatitis C were: physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and blood donation in 6.02% (n=5). Mentioned a predominance of dental care in 68.4% [12], this mode of contamination is rarely incriminated in certain countries such as Tunisia (0.63%) [13]. History of surgery was noted at 24.09 % of cases. These antecedents may be susceptible to a source of contamination and this could be explained by the fact of the use of reusable (non-disposable) materials and the fact that the instrument sterilization measures were not sufficiently effective to eliminate the HCV. The causal link with the surgical procedure cannot be established with certainty. In a Tunisian study, the history of surgical intervention was noted in 20% of cases [13]. The transfusion of blood and its derivatives found in 12.04% in our series. At the international level, the transfusion of blood products and derivatives played a major role in the spread of HCV infection until 1990. According to a study carried out by a French team in 2005, the suspected risk factors were: transfusion was responsible for chronic HCV infection in France 38.8%., intravenous drug use (21%), nasal drug use (6.2%), occupational exposure (5, 5%), nosocomial exposure (22.6%) and no risk factors found in 14.7% [14]. Likewise, DIALLO MS et al.
[9] noted blood transfusion in 4.5% of cases and dental care in 4% of cases as the main risk factors. In Benin KPOUSSOU AR et al.
[7] found scarification as the predominant risk factor with a frequency of 55.1%. As was demonstrated in our study, tattoos (8.43) and scarifications (7.22%) played a significant role in the transmission of HCV. Finally, no risk factors were found in 33.73% of our patients (Table 1). For other authors, the mode of contamination could not be identified in 8 to 20% of cases [13]. In these patients, either there is an as yet unknown mode of contamination or it is more likely an unknown percutaneous transmission: mass vaccination, scarification, circumcision, piercing, sharing of personal hygiene objects, intra- family, injection of medication using non-disposable equipment. These situations are present in our country with limited resources. The circumstances of discovery of viral hepatitis C were: physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and blood donation in 6.02% (n=5). Mentioned a predominance of dental care in 68.4% [12], this mode of contamination is rarely incriminated in certain countries such as Tunisia (0.63%) [13]. History of surgery was noted at 24.09 % of cases. These antecedents may be susceptible to a source of contamination and this could be explained by the fact of the use of reusable (non-disposable) materials and the fact that the instrument sterilization measures were not sufficiently effective to eliminate the HCV. The causal link with the surgical procedure cannot be established with certainty. In a Tunisian study, a history of surgical intervention was noted in 20% of cases [13]. Blood transfusion and its derivatives were found in 12.04% in our series. At the international level, the transfusion of blood products and derivatives played a major role in the spread of HCV infection until 1990. According to a study carried out by a French team in 2005, the suspected risk factors were: transfusion was responsible for chronic HCV infection in France 38.8%., intravenous drug use (21%), nasal drug use (6.2%), occupational exposure (5, 5%), nosocomial exposure (22.6%) and no risk factors found in 14.7% [14]. Likewise, DIALLO MS et al. [9] noted blood transfusion in 4.5% of cases and dental care in 4% of cases as the main risk factors. In Benin KPOUSSOU AR et al. [7] found scarification as the predominant risk factor with a frequency of 55.1%. As was demonstrated in our study, tattoos (8.43) and scarifications (7.22%) played a significant role in the transmission of HCV. Finally, no risk factors were found in 33.73% of our patients (Table 1). For other authors, the mode of contamination could not be identified in 8 to 20% of cases [13]. In these patients, either there is an as yet unknown mode of contamination or it is more likely an unknown percutaneous transmission: mass vaccination, scarification, circumcision, piercing, sharing of personal hygiene objects, intra- family, injection of medication using non-disposable equipment. These situations are present in our country with limited resources. The circumstances of discovery of viral hepatitis C were: physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and blood donation in 6.02% (n=5). These antecedents may be susceptible to a source of contamination and this could be explained by the fact of the use of reusable (non- disposable) materials and the fact that the instrument sterilization measures were not sufficiently effective to eliminate the HCV. The causal link with the surgical procedure cannot be established with certainty. In a Tunisian study, the history of surgical intervention was noted in 20% of cases [13]. The transfusion of blood and its derivatives found in 12.04% in our series. At the international level, the transfusion of blood products and derivatives played a major role in the spread of HCV infection until 1990. According to a study carried out by a French team in 2005, the suspected risk factors were: transfusion was responsible for chronic HCV infection in France 38.8%., intravenous drug use (21%), nasal drug use (6.2%), occupational exposure (5, 5%), nosocomial exposure (22.6%) and no risk factors found in 14.7% [14]. Likewise, DIALLO MS et al. [9] noted blood transfusion in 4.5% of cases and dental care in 4% of cases as the main risk factors. In Benin KPOUSSOU AR et al. [7] found scarification as the predominant risk factor with a frequency of 55.1%. As was demonstrated in our study, tattoos (8.43) and scarifications (7.22%) played a significant role in the transmission of HCV. Finally, no risk factors were found in 33.73% of our patients (Table 1). For other authors, the mode of contamination could not be identified in 8 to 20% of cases [13]. In these patients, either there is an as yet unknown mode of contamination or it is more likely an unknown percutaneous transmission: mass vaccination, scarification, circumcision, piercing, sharing of personal hygiene objects, intra- family, injection of medication using non-disposable equipment. These situations are present in our country with limited resources. The circumstances of discovery of viral hepatitis C were: physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and blood donation in 6.02% (n=5). These antecedents may be susceptible to a source of contamination and this could be explained by the fact of the use of reusable (non-disposable) materials and the fact that the instrument sterilization measures were not sufficiently effective to eliminate the HCV. The causal link with the surgical procedure cannot be established with certainty. In a Tunisian study, the history of surgical intervention was noted in 20% of cases [13]. The transfusion of blood and its derivatives found in 12.04% in our series. At the international level, the transfusion of blood products and derivatives played a major role in the spread of HCV infection until 1990. According to a study carried out by a French team in 2005, the suspected risk factors were: transfusion was responsible for chronic HCV infection in France 38.8%., intravenous drug use (21%), nasal drug use (6.2%), occupational exposure (5, 5%), nosocomial exposure (22.6%) and no risk factors found in 14.7% [14]. Likewise, DIALLO MS et al. [9] noted blood transfusion in 4.5% of cases and dental care in 4% of cases as the main risk factors. In Benin KPOUSSOU AR et al. [7] found scarification as the predominant risk factor with a frequency of 55.1%. As was demonstrated in our study, tattoos (8.43) and scarifications (7.22%) played a significant role in the transmission of HCV. Finally, no risk factors were found in 33.73% of our patients (Table 1). For other authors, the mode of contamination could not be identified in 8 to 20% of cases [13]. In these patients, either there is an as yet unknown mode of contamination or it is more likely an unknown percutaneous transmission: mass vaccination, scarification, circumcision, piercing, sharing of personal hygiene objects, intra- family, injection of medication using non-disposable equipment. These situations are present in our country with limited resources. The circumstances of discovery of viral hepatitis C were: physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and blood donation in 6.02% (n=5). Transfusion was responsible for chronic HCV infection in France 38.8%, intravenous drug use (21%), nasal drug use (6.2%), occupational exposure (5.5%), nosocomial exposure (22.6%) and no risk factors found in 14.7% [14]. Likewise, DIALLO MS et al. [9] noted blood transfusion in 4.5% of cases and dental care in 4% of cases as the main risk factors. In Benin KPOUSSOU AR et al. [7] found scarification as the predominant risk factor with a frequency of 55.1%. As was demonstrated in our study, tattoos (8.43) and scarifications (7.22%) played a significant role in the transmission of HCV. Finally, no risk factors were found in 33.73% of our patients (Table 1). For other authors, the mode of contamination could not be identified in 8 to 20% of cases [13]. In these patients, either there is an as yet unknown mode of contamination or it is more likely an unknown percutaneous transmission: mass vaccination, scarification, circumcision, piercing, sharing of personal hygiene objects, intra-family, injection of medication using non-disposable equipment. These situations are present in our country with limited resources. The circumstances of discovery of viral hepatitis C were: physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and blood donation in 6.02% (n=5). Transfusion was responsible for chronic HCV infection in France 38.8%, intravenous drug use (21%), nasal drug use (6.2%), occupational exposure (5.5%), nosocomial exposure (22.6%) and no risk factors found in 14.7% [14]. Likewise, DIALLO MS et al. [9] noted blood transfusion in 4.5% of cases and dental care in 4% of cases as the main risk factors. In Benin KPOUSSOU AR et al. [7] found scarification as the predominant risk factor with a frequency of 55.1%. As was demonstrated in our study, tattoos (8.43) and scarifications (7.22%) played a significant role in the transmission of HCV. Finally, no risk factors were found in 33.73% of our patients (Table 1). For other authors, the mode of contamination could not be identified in 8 to 20% of cases [13]. In these patients, either there is an as yet unknown mode of contamination or it is more likely an unknown percutaneous transmission: mass vaccination, scarification, circumcision, piercing, sharing of personal hygiene objects, intra- family, injection of medication using non-disposable equipment. These situations are present in our country with limited resources. The circumstances of discovery of viral hepatitis C were: physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and blood donation in 6.02% (n=5).physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and donation of blood in 6.02% (n=5).physical asthenia (43.37% n= 36), cytolysis and jaundice in 36.14% (n=30), during voluntary screening in 14.46% (n=12) and donation of blood in 6.02% (n=5).
In Benin KPOUSSOU AR et al. [7] noted the main circumstances of discovery were the revelation by a symptom (38%), systematic screening (23%) for viral hepatitis and respectively 48.1% and 26.1% for viral hepatitis B.
In our series, the serum aminotransferase (ALT) dosage showed ALT lower than normal in 54%; ALT between one and three times normal in 30% and ALT greater than three times normal in 16% (figure 1). Serum aminotransferases marks of hepatocyte lysis constitute a fundamental element in the biological diagnosis of viral hepatitis, transaminases also represent an element in monitoring viral hepatitis C. Their increase for more than six months signals the transition to chronicity and 60 to 90% of chronic hepatitis cases progress to fibrosing liver disease, unlike patients with normal transaminases [15,16]. This high frequency of cytolysis in our study could be explained by the fact that in Black Africa the causes of hepatic cytolysis are numerous, intricate and reminiscent of viral, drug and toxic causes [17].
In terms of evaluating hepatic fibrosis; pulse elastometry showed absence of fibrosis or minimal fibrosis (F0F1) in 36.14%, moderate fibrosis (F2) in 30.12%; severe fibrosis (F3) in 21.69% and cirrhosis (F4) in 12.05%. As for Diallo MS et al. in their study they reported liver fibrosis with the following frequencies: 58.82% had absence of fibrosis or minimal fibrosis (F0F1), 22.94% moderate fibrosis (F2), 11.76% severe fibrosis (F3 ) and 6.47% fibrosis classified F4 [18].
Likewise in another of their series in 2024, DIALLO MS et al. [9] noted a distribution of hepatic fibrosis using Fibroscan as follows: 51% (n=102) had no fibrosis or minimal fibrosis (F0F1), 30% (n=60) with moderate fibrosis (F2), 17.5% (n=35) severe fibrosis (F3) and 2.5% (n=5) fibrosis classified F4.
Morphologically, abdominal ultrasound was normal in 80% of cases, hepatic steatosis in 10% of cases, biliary cysts in two patients, cirrhosis in six cases with nodules suspicious for HCC in three patients. Portal trunk dilatation in two patients. CT scan found the kinetics of HCC with “Wash out” in these three patients. This result is close to that reported by SOMBIE R et al. in Burkina Fasso, [11] who noted a normal ultrasound in 63% of cases, hepatic steatosis in 21.7% of cases, cirrhosis in four cases including three complicated by CHC. In their study, the scan carried out in three patients revealed one case of cirrhosis and two cases of HCC. This result is superimposable to that of DIALLO MS et al. [9] who noted in their series, abdominal ultrasound found: a normal liver in 75%, hepatic steatosis in 20%, a non-decompensated cirrhotic liver in 5%.
It was noted that 56% of patients had a viral load (HCV RNA) less than 50,000 IU/mL and 44% had a viremia greater than 50,000 IU/mL. Three of our patients had hepatitis B surface antigen and two patients had HCV and HIV co-infection. Several French collaborative studies, carried out under the aegis of GERMIVIC, from a cohort of approximately 25,000 HIV-infected patients have reported 20% HCV-HIV co-infection, this high rate can be explained by the fact that the modes of transmission are common, especially drug addiction and unprotected sexual intercourse [19].
Conclusion
The prevalence of viral hepatitis C in our series is 3.70%. Viral hepatitis C remains a public health problem, given its complications with the risk of progression to cirrhosis and hepatocellular carcinoma. Men are more infected. As the treatment is very expensive, it is not equally accessible to everyone. Emphasis must be placed on avoiding contamination risk factors. Our States must promote health insurance to the entire population without exclusion.
Declaration for Human Rights
The hospital has consented to the use of data from patients who have been consulted in the department. This study was approved by the hospital ethics committee and the principles of the Declaration of Helsinki were followed.
References
- Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern 2012; 156: 271-278.
- Kodjoh Fighting viral hepatitis B and C in Africa Focus on Benin. Med Sante Trop. 2015; 25: 131-134.
- Guéchot Evaluation of hepatic fibrosis. Rev Fr Lab. 2003; 358: 39-43.
- Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral WHO. 2016.
- Diallo M Sarifou, Youssouf Oumarou, Diallo A Tidiane, et Risk Factors for Hepatic Fibrosis in Patients with Chronic Viral Hepatitis B at the Hospital National Donka CHU in Conakry. Gastroint Hepatol Dig Dis. 2024; 7: 1-6
- National strategic plan to combat viral hepatitis in Guinea, NO1/MS/SGG of August 30. 2018: 91.
- Kpoussou AR, Séhonou J, Wanvoegbe FA, et Viral hepatitis B and C: Epidemiological and clinical aspects at the National Hospital and University Center of Cotonou. Black African Medicine. 2019; 66: 6-12.
- Global surveillance and control of hepatitis C; Report of a WHO consultation organized in collaboration with the Viral Hepatitis Prevention Board. J Viral Hepatitis 1999; 6: 35-47.
- Diallo M Sarifou, Youssouf Oumarou, Pare Stella Line E, et al. Cirrhosis and Its Complications at the Donka National Hospital of Gastroint Hepatol Dig Dis. 2024; 7: 1-7.
- Hadziyannis SJ, Sette H Jr, Morgan TR, et A randomized style of treatment duration and ribavirin dose. Ann Intern Med. 2004; 140: 346-355.
- Sombie R, Bougouma A, Somda S, et Chronic viral hepatitis C: epidemiology, diagnosis and treatment at Yalgado-Ouédraogo University Hospital in Ouagadougou. J. Afr. Hepatol Gastroenterol. 2011; 5: 6-13.
- Couzigou P, Castera L. side effects of treatment and quality of In Marcellin P. Asselah T. Viral hepatitis Wolters Kluwer France SQS. 2008; 191-211.
- Ben Alaya, Bouafif N, triki H, mejri s, et al. A case control method to assess risk factors for Hepatitis C among a general population in a highly endemic area of northwest Tunisia. Arch Inst Pasteur 2007; 84: 21-27.
- Poupon R. State of knowledge on hepatitis C: epidemiology, management and current treatments. Bull Acad Natle Méd. 2005; 189: 375-387.
- Miailhes P, Trepo C. The natural hottopy of hepatitis C virus Med Mal Inft. 2000; 30: 8-13.
- Buffet Serum biological markers and screening for viral hepatitis. Rev Prat. 1995; 45: 168-173.
- Diallo MS, Wann TA, Diallo D, et al. Autoimmune hepatitis complicated by cirrhosis: about an observation at the Conakry University Jaccr Africa. 2023; 7: 65-70.
- Diallo MS, Youssouf O, Yaogo A, et Evaluation of Hepatic Fibrosis and Hepatic Steatosis by Pulse Elastography (FIBROSCAN) in Asymptomatic Patients about 170 Cases at the Donka CHU National Hospital in Conakry. Open Journal of Gastroenterology. 2024; 14: 125-138.
- Cacouba C, Rosenthal E. Mono-infection with hepatitis C virus (HCV) and co-infection with human immunodeficiency virus and HCV: comparative analysis of treatment based on two large French surveys Elsevier. 2012; 33: 355-357.