Gluten Fibromyalgia
Author'(s): Dmitriy A. Labunskiy1* and Svetlana V. Kopishinskaya2
1 University of Northern California, Petaluma, CA, United States.
2 Department of Neurology, Nizhny Novgorod State Medical Academy Nizhny Novgorod, Russian, Russia.
*Correspondence:
Dmitriy Labunskiy, University of Northern California, Petaluma,CA, United States, E-mail: Dlabunskiy@hotmail.com.
Received: 22 December 2017; Accepted: 07 January 2018
Citation: Liliane K. Siransy, Chiayé C.A. Yapo-Crézoit, Maxime K. Diane, et al. Th3 and Th2 Cytokines Pattern among Sickle Cell Disease Patients in Cote d’ivoire. Clin Immunol Res. 2018; 2(1): 1-4.
Keywords
Introduction
Fibromyalgia (FM) is a complex chronic pain syndrome that affects about 2% of the population, mainly women, from all over the world, and is characterized by widespread pain in soft tissues, generalized sensory points, pathological fatigue and sleep disturbances. The prevalence of FM in different countries ranges from 2% in the US and France to 4% in Spain [1]. FM is characteristic for all ages, all ethnic groups and cultures. Despite the fact that there are no sex differences in childhood, at the age of 50-60 years, FM occurs seven times more often in women [2].
Although the etiology of FM is still not clear, there has been a violation of the processing of pain, depending on neuroendocrine, neurotransmitter and neurosensory disorders [3]. These anomalies are more common in genetically predisposed people [4,5].
The pathogenesis of this disease remains unclear, and at the moment there is no method of examination confirming FM. Early studies of the pathogenesis of FM were focused on the possible role of muscles and connective tissue. However, no changes in the muscles could not be detected.
To diagnose FM it is necessary that there be spontaneous chronic pain, which should be in all four limbs and the trunk [6]. Pain is often described as persistent, diffuse, deep, aching, throbbing, sometimes with piercing sensations in the muscles; It can be repetitive, but often constant with periodic increase. The pain can be so intense that the patient is unable to perform his daily activities. Characterized by the presence of increased pain sensitivity at certain points [7,8]. They can be detected either manually or using an algometer. In addition to pain in FM, there are also other symptoms: chronic insomnia, nocturnal myoclonus and bruxism, morning stiffness in the morning, increased fatigue during the day, physical weakness, cognitive impairment, complaints of memory loss [9,10]. Often there is numbness, tingling and dysesthesia in the hands and feet, throbbing pain in the nape, dizziness, fainting, abdominal or pelvic pain, diarrhea, constipation, dysuria. There are works where the supposed role of polyneuropathy of fine fibers in the pathogenesis of FM [11,12] is indicated. Almost half of patients with FM exhibit depression and/or anxiety.
The contribution of FM to deterioration in the quality of life of patients is comparable to rheumatoid arthritis. Presumably up to 40 million people around the world suffer from FM [13]. The establishment of the correct diagnosis of FM and optimal treatment according to the prospective five-year study results in a statistically significant improvement in the quality of life of the patient, and according to the results of the 15-year retrospective study, there is a significant decrease in both visits to physicians of various specialties, and the appointment of diagnostic manipulations and drugs [14].
FM is not considered an autoimmune disease, although it often combines with autoimmune diseases. For example, 30% of women with Bechterew's disease, 16% with systemic lupus erythematosus, 15% with rheumatoid arthritis have violations that meet the criteria of FM. FM is also often detected in 34% of patients with hypothyroidism, 26% with Crohn's disease, 11% with ulcerative colitis [15,16]. The relationship between FM and gastrointestinal tract diseases was first described by M. Yunus with co-authored in 1981 [17]. In FM, irritable bowel syndrome (IBS), chronic fatigue syndrome and musculoskeletal pain are common. It is known that before the pain syndrome of FM appears, many patients have complaints of abdominal pain, constipation, diarrhea. There is evidence that FM often occurs in women with gastrointestinal pathology, for example, with celiac disease, IBS, Crohn's disease, ulcerative colitis [15].
Celiac disease is a multisystem autoimmune disease that occurs as a result of gluten intolerance and affects 1-2% of the population, mostly women [18]. Patients with celiac disease carry carriers of one of the two HLA-II genotypes, DQ2 and DQ8. Gliadin peptides induce an aberrant immune response and lead to production of antibodies to tissue transglutaminase and to immune chronic inflammation of the small intestinal mucosa [19]. This inflammation is characterized by atrophy of the villi, intraepithelial lymphocytosis and crypt hyperplasia. A clinical manifestation of celiac disease can occur at any age with intestinal and / or extraintestinal symptoms [20-22].
Celiac disease often remains undiagnosed. It is known that celiac disease is seven times more common in IBS compared with the general population [7,23,24]. Testing of patients with IBS on celiac disease is recommended in foreign clinical practice [25-27]. Rodrigo L. and colleagues in 2013 conducted a survey for celiac disease in 104 patients with IBS and FM and revealed 6.7% of patients with celiac disease [28]. At the same time, according to Tovoli F., who examined 90 patients with FM for celiac disease and found only 1% of patients with celiac disease, as in the general population [29]. It is known that from 20 to 32% of patients with IBS have FM, and from 32 to 70% of patients with FM suffer from IBS [24,26].
Based on clinical data, the patients of the main group were divided into patients with a typical and atypical form of celiac disease. The typical form of celiac disease was 63.5%, atypical - 36.5% of the total number of patients, the given ratio is consistent with the data of a number of authors [2,26,27]. The distribution of men and women in the main group in terms of the form of celiac disease was not statistically significant.
As a comparison group, persons diagnosed with reflux esophagitis without celiac disease (118 patients) were used, which was determined by histological examination of biopsy specimens of the duodenal mucosa. The comparison group was balanced against the main group by sex and age using the "copy-pair" method and 100 observations were included in the final analysis. The shares of men and women - 30% and 70% - did not differ in the compared groups. The mean age in the main group was 50.5 (39.0, 64.0) years, in the comparison group - 51.5 (40.5, 63.0) years (p = 0.732). Comparison of the frequency distribution of observations in the compared groups as a function of age was not statistically significant (p = 0.809).
Statistical processing was carried out with the help of a specialized software package SPSS, V. 17.0. For descriptive statistics, the mean values (Me - median) were calculated in the form Me (P25; P75), where P25 and P75 are the lower and upper quartiles, and the relative indices (P, in%) in the form P ± m, where m is the error of the relative The indicator, at zero frequency of the studied indicator - in the form of P (the lower level, the upper level of 95% confidence interval based on the exact Clopper-Pearson method). Determination of the correspondence between the type of distribution of the characteristic to the normal was carried out using the Shapiro-Wilk and Kolmogorov-Smirnov criteria with the Lillie force correction.
In cases of comparing groups by the values of individual characteristics for unrelated samples, a chi-square test was used to compare qualitative data Pearson or Fisher's exact test (for four- field tables), for quantitative data - Mann-Whitney U-test. The use of nonparametric methods was dictated by the observed limitations in the use of their parametric analogs. The critical threshold of statistical significance was determined at the level of p <0.05.
The patient of each group was tested for the presence of sensitive points according to the criteria of the American College of Rheumatology from 1990 [8]. Sensitive points were detected by palpation with a pressure force of 4 kg. Each point was palpated for 4 seconds. The patient answered "yes" or "no" if he felt or did not have any pain. When the patient answered "yes" at 11 or more points, the doctor asked to assess the pain level on a visual analog scale (VAS) from 0 to 100 mm.
Each patient independently filled a Russian version of the questionnaire on FM "Revised Fibromyalgia Impact Questionnaire (FIQR)". This questionnaire consisted of 10 items and was estimated from 0 to 80 points. The degree of severity of FM was estimated by points: up to 15 points - no FM, 16 - 49 points - light FM, 50 - 64 - moderate FM and 65 - 80 - heavy FM.
In all patients of both groups, electroneuromyographic parameters were recorded on the apparatus "MBN-neuromyograph" (Russia). The electroneuromyographic study was carried out using a stimulating surface plate electrode (the cathode was located distally, the anode was proximal), and the outflow was a standard set of unipolar, plate-like electrodes with a diameter of 5 mm.
The motor portions of the median and peroneal nerves were studied and the following were evaluated: the amplitude of the motor response (M-response), the M-response form, the duration of the M-Response, M-response area, M-response latency, pulse rate (SPI), residual latency (RL), and sensory portions of the median nerves and gastro-cnemious were examined using the antidromic technique (distal overlapping electrodes and stimulation similar to SPI in motor Fibers) and evaluated: the amplitude of the action potential (PD), the shape of the PD, the duration of the PD, the area of the PD, the latency of the PD, the SPI on the sensor fibers.
For most patients of both groups without pathology, according to electroneuromyography, three skin samples were taken from the distal portion of the lower extremity (100 mm above the lateral malleolus), followed by immunohistochemical examination of the skin biopsy specimen by C-fibers with antibodies to the protein gene product 9.5 (protein gene product 9.5 - PGP 9.5). The study of the drugs was carried out by light microscopy, during which the linear density of intraepidermal thin nerve fibers was determined. Patients completed the Severity Score Class of Restless Legs Syndrome (RLS), published in 2003 by the International Restless Legs Syndrome Study Group, where 1-10 scores mean easy RLS,11-20 mild, 21-30 - pronounced, 31-40 - strongly pronounced.
Statistically significant differences in both groups were found by the presence of polyneuropathy of fine fibers from skin biopsy data (p = 0.005). By such signs as the number of sensitive points, the duration of FM in months, the number of points on the questionnaire FIQR, the presence of polyneuropathy of thick fibers by Data of electroneuromyography, presence of RLS, anxiety, depression were absent (p>0.05, Table 1). It is statistically significant that the pain syndrome according to YOUR scale in FM in patients with celiac disease was more pronounced than in the comparison group (p = 0.006). Women with celiac disease and FM had more sensory points than men of the same group (p = 0.003), but the mean duration of FM was 4.2 shorter (p = 0.002, Table 2).
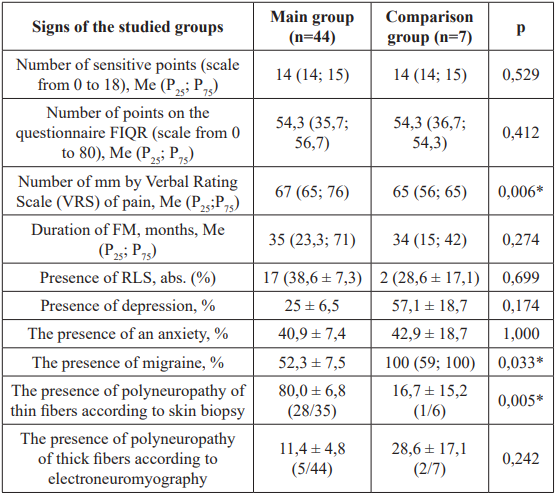
Table 1: Characteristics of fibromyalgia patients with the compared groups
Note: *: The differences are statistically significant (p<0.05) according to the bilateral Mann-Whitney test, FIQR: The questionnaire on Fibromyalgia "Revised Fibromyalgia Impact Questionnaire"; VRS: Verbal Rating Scale; FM: Fibromyalgia; RLS: Restless Legs Syndrome.
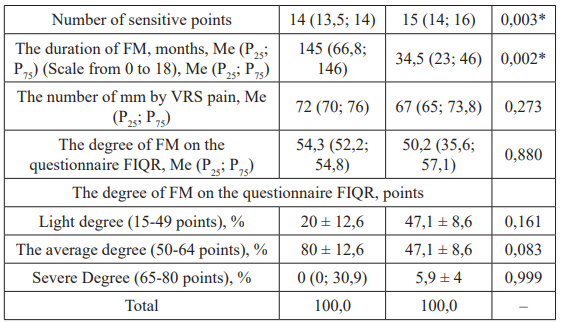
Table 2: Comparison of the parameters in patients with fibromyalgia of the main group as a function of sex, Me (P25; P75).
Note: *: The differences are statistically significant (p <0.05) by the bilateral Mann-Whitney test, FIQR: the questionnaire on fibromyalgia "Revised Fibromyalgia Impact Questionnaire"; VRS: Visual Raiting Scale; FM: Fibromyalgia; RLS: Restless Legs Syndrome.
The relationship of individual parameters with the presence of FM in patients of the main group and the comparison group was characterized by the presence of similar traits and differences (Table 3). In particular, there was a coincidence of the fact and direction of the relationship of FM and parameters such as RLS, depression, anxiety. The relationship of FM with polyneuropathy of thin and thick fibers was noted only in patients with celiac disease, and the relationship of FM with migraine only in the comparison group.
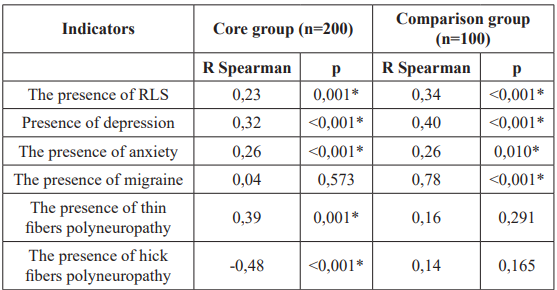
Table 3: Interrelation of individual indicators with the presence of fibromyalgia in patients of the main group and the comparison group (the value of the Spearman correlation coefficient R and its significance, p). Note: *: The correlation has a statistical significance (p≤0,05); RLS: Restless Leg Syndrome.
Discussion
The study showed that the prevalence of FM in patients with celiac disease is three times higher than in the population. The typical form of celiac disease and the age of patients 40-59 years are prognostic unfavorable signs of the development of FM in patients with celiac disease. Qualitative signs of FM in patients with celiac disease did not have any specific features: statistically significant differences in both groups by such features as the number of sensitive points, the duration of FM in months, the number of points on the FM questionnaire "Revised Fibromyalgia Impact Questionnaire" were absent (p> 0.05). Women with celiac disease and FM had more sensory points than men in the same group. The average duration of FM was much shorter in women with celiac disease than in men. According to our research, the dependence of FM on polyneuropathy of fine fibers in patients with celiac disease is important in terms of the pathogenesis of FM.
The findings allow us to consider that a gluten-mediated autoimmune inflammatory process, beginning in the digestive tract in patients with celiac disease, can lead to a central Sensitization, and, therefore, to the development of FM. The development of central sensitization in patients with celiac disease is possible as a result of a large flow of painful impulses with concomitant polyneuropathy of fine fibers, as well as a violation of descending pain inhibition.
Conclusion
Both FM and celiac disease are often undiagnosed diseases, typical of any gender and age. FM is often combined with diseases of the gastrointestinal tract. In this study, the frequent occurrence of FM in patients with celiac disease was identified. It is necessary to exclude FM in patients with celiac disease. The dependence of FM on polyneuropathy of fine fibers, restless leg syndrome, depression, anxiety in the group of patients with celiac disease was revealed. One of the modern methods for diagnosing polyneuropathy of fine fibers in patients with FM is the immunohistochemical study of skin biopsy specimens on C-fibers by means of antibodies to the protein gene product 9.5.
References
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr. Pain Headache 2013; 17: 356-367.
- Tabeeva Fibromyalgia the symptoms formation and principles of the therapy. Neurology, neuropsychiatry and psychosomatics. 2012; 1: 23-27.
- Esin RG, Esin OR, Muhametova JeR, et al. Syndromes of central Neurologichesky Vestnik. Journal named after VM. Bekhterev. 2013; 3: 64-70.
- Smith HS, Harris R, Clauw Fibromyalgia an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician. 2011; 14: 217-245.
- Sarzi-Puttini P, Atzeni F, Di Franco M, et al. Dysfunctional syndromes and fibromyalgia a 2012 critical Clin. Exp. Rheumatol. 2012; 30: 143-151.
- Danilov Diagnostics and treatment of fibromyalgia. Attending doctor. 2012; 5: 30-34.
- Rodionova ON, Babaeva AR. Conjugation of fibromyalgia and functional disorders of the gastrointestinal tract. Preventive and clinical medicine. 2011; 4: 102-105.
- Wolfe F, Smythe HA, Yunus MB, et The American College of Rheumatology 1990 criteria for classification of fibromyalgia report of the Multicenter Criteria Committee. Arthritis Rheum. 1990; 33: 160-172.
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010; 62: 600-610.
- Lubart E, Leibivitz A, Shapir V, et al. On-spot rheumatology consultations in a multilevel geriatric Isr. Med. Assoc. J. 2014; 16: 33-36.
- Kopishinskaya SV. Gluten pain syndromes. Abstracts of the 14th World Congress on Pain, 2012; 144.
- Levine TD, Saperstein DS. Routine use of punch biopsy to diagnose small fiber neuropathy in fibromyalgia Clin. Pheumatol. 2014.
- Tabeeva GR. Fibromyalgia. Sechenovskiy vestnik. 2014; 1: 40-46.
- Clark P, Paiva ES, Ginovker A, et A patient and physician survey of fibromyalgia across Latin America and Europe. BMC Musculosceletal Disord. 2013; 14: 188-217.
- Yunus MB. The prevalence of fibromyalgia in other chronic pain Pain Res. Treat. 2012; 12: 584-573.
- Mohammad A, Carey JJ, Storan E, et Prevalence of fibromyalgia among patients with chronic hepatitis C infection: relationship to viral characteristics and quality of life. J. Clin. Gastroenterol. 2012; 46: 407–412.
- Yunus B, Masi AT, Calabro JJ, et al. primary fibromyalgia fibrositis clinical study of 50 patients with matched normal controls. Semin. Arthritis Rheum. 1981; 11: 151-171.
- Crowe Celiac disease. Ann. Intern. Med. 2011; 154: 5-16.
- Krums LM, Parfenov AI, Sabelnikova EA, et al. Treatment and prophylaxis of the glutensensitive celiac Experimental and clinical gastroenterology. 2011; 2: 86-92.
- Parfenov AI. Glutensensitive celiac disease multidisciplinary human Verhnevolzhskiy medical journal. 2013; 11: 42-48.
- Rodrigo L, Blanco I, Bobes J, et Clinical impact of a gluten- free diet on health-related quality of life in seven fibromyalgia syndrome patients with associated celiac disease. BMC Gastroenterology. 2013; 13: 157-166.
- Taubman B, Mamula P, Sherry Prevalence of asymptomatic celiac disease in children with fibromyalgia. Pediatric Rheumatology. 2011; 9: 11-14.
- Jadallah KA, Khader YS. Celiac disease in patients with presumed irritable bowel syndrome: a case-finding World J. Gastroenterol. 2009; 15: 5321-5325.
- Sainsbury A, Sanders DS, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: a meta-analysis. Clin. Gastroenterol. Hepatol. 2013; 11: 359-365.
- Spiegel BM, Farid M, Esrailian E, et al. Is irritable bowel syndrome a diagnosis of exclusion a survey of primary care providers, gastroenterologists, and IBS experts. Am. J. 2010; 105: 848-858.
- Ford AC, Chey WD, Talley NJ, et al. Yield of diagnostic tests for celiac disease in individuals with symptom suggestive of irritable bowel syndrome: systematic review and meta- Arch. Intern. Med. 2009; 169: 651-658.
- ZwolinskaWcislo M, Galicka-Latala D, Rozpondek P, et al. Frequency of celiac disease and irritable bowel syndrome coexistence and its influence on the disease course. Przegl. 2009; 66: 126-129.
- Rodrigo L, Blanco I, Bobes J, et Remarkable prevalence of celiac disease in patients with irritable bowel syndrome plus fibromyalgia in comparison with those with isolated irritable bowel syndrome: a case-finding study. Arthritis Res. Therapy. 2013; 15: 1-12.
- Tovoli F, Giampaolo L, Caio G, et al. Fibromyalgia and coeliac disease a media hype or an emerging clinical problem Exp. Rheumatol. 2013; 31: 50-52.