Haptic Vibrotactile Trigger Technology: Disrupting the Neuromatrix to Reduce Pain Severity and Interference: Results from the HARMONI Study
Author'(s): Jeffrey Gudin1, Derek Dietze2, Galen Dhaliwal3 and Peter Hurwitz4*
1University of Miami School of Medicine, Miami, Florida USA
2Metrics for Learning LLC, Queen Creek, Arizona, USA.
3Toronto, Canada.
4Clarity Science LLC, Narragansett, Rhode Island, USA.
*Correspondence:
Peter Hurwitz, Clarity Science LLC, 750 Boston Neck Road, Suite11, Narragansett, RI 02882, Tel +1917 757 0521, Fax +1855-891-8303
Received: 20 Nov 2022; Accepted: 20 Dec 2022; Published: 30 Dec 2022
Citation: Gudin J, Dietze D, Dhaliwal G, et al. Haptic Vibrotactile Trigger Technology: Disrupting the Neuromatrix to Reduce Pain Severity and Interference: Results from the HARMONI Study. Anesth Pain Res. 2022; 6(2): 1-7.
Abstract
The prevalence of pain and pain-related diseases are so vast that they are the leading causes of disability and disease burden across the globe. Over 100 million people are estimated to live with chronic or recurrent pain in the United States and it is the most common reason patients consult primary care clinicians. Conventional pharmacological treatments for pain have been associated with dangerous adverse effects. Identifying effective, alternative treatment strategies, including those that are non-invasive and non-pharmacologic and that have reduced and limited side effect profiles, will provide options that may be preferable in how clinicians traditionally treat pain. Understanding the pain neuromatrix may assist in identifying these alternative approaches that reduce pain severity and interference, and that improve patient outcomes.
The neuromatrix of pain is a network of neuronal pathways and circuits responding to sensory (nociceptive) stimulation. Researchers have shown that these pathways and areas of the brain that are associated with the neuromatrix can change in response to external stimuli. Haptic vibrotactile trigger technology (VTT) is designed to target the nociceptive pathways and theorized to disrupt the neuromatrix of pain. The technology has been incorporated into non-invasive, non-pharmacological topical patches and other routes of delivery.
The purpose of this IRB-approved, minimal risk study was to evaluate patients’ experiences and/or perceptions and patient response for those who received a haptic vibrotactile trigger technology (VTT) embedded non-pharmacologic, non-invasive, over-the-counter pain patch (FREEDOM Super Patch with VTT; Srysty Holding Co, Toronto, Canada).
Methods: Baseline, 7- and 14-day data were recorded in one hundred forty-eight (148) adult subjects (96 females and 52 males) with a mean age of 53 years who presented with mild, moderate and even severe musculoskeletal, arthritic and neurological pain. The study evaluated changes in overall severity and interference scores via a validated scale (Brief Pain Inventory (BPI)), changes in the use of prescription and OTC medications, patient satisfaction, and any side effects reported while using the patch. Future analyses will compare the outcomes reported here with non-active control and crossover treatment groups.
Results: The results showed statistically significant decreases in mean BPI severity and interference scores after using the VTT embedded pain patch. After 14 days, the vast majority of patients reported “less” or “a lot less” usage of oral medications and were very/extremely satisfied with the patch. Results also showed statistically significant and positive outcomes in all measured Quality of Life (QoL) components with improvements in general activity, mood, relations with other people, sleep, normal work, walking ability, and enjoyment of life.
Conclusions: Study results indicate that this non-pharmacologic, non-invasive, haptic vibrotactile trigger technology (VTT) embedded topical patch reduces pain severity and interference scores and may reduce the use of concurrent medications, including prescribed anti-inflammatory and other oral medication for adult patients with arthritic, neuropathic, and musculoskeletal pain. Results reported suggest that the non-pharmacological topical pain patch has incredible potential to be added to the current arsenal of noninvasive and nonpharmacological pain therapies.
Keywords
Introduction
Worldwide, pain and pain-related diseases are the leading causes of disability and disease burden. In the United States, pain is the most common reason patients consult primary care providers and an estimated 100 million people live with pain everyday [1]. Acute, chronic, and mild to moderate pain issues are widely prevalent throughout the US and have been shown to impact quality of life and activities of daily living (ADLs) [2-4].
In recent years, several medical associations have updated their guidelines for pain management and recommend a multi-modal approach that includes non-invasive and non-pharmacological therapies as a first line treatment before consideration of other approaches [5,6]. There has been an effort to minimize the use of pharmacologic treatments in light of their potential adverse effects and toxicities. As we progress, it is important to investigate novel nonpharmacologic treatment options for patients as part of a multi- modal treatment approach to maximize effectiveness, improve a patient’s quality of life (QoL), and restore function. A variety of non-pharmacologic treatments have been reported to be successful in addressing a patient’s pain with limited, if any, side effects. These include physical therapeutic, behavioral, and topical drug and device therapies [7-9]. Evidence supports that topical analgesic therapies are safe and effective for pain conditions and should be considered as part of a multi-modal treatment strategy [10,11].
Several theoretical frameworks have been proposed to explain the physiological basis of pain, the most well-known of which is the Gate Control Theory [12]. Over the past several years, researchers have developed an understanding of the Neuromatrix Theory of Pain (NTP) through imaging studies and related theories of how various peripheral, spinal and brain regions modulate and perceive pain [13-15]. By extension of the so-called “gate control” theory of pain, the neuromatrix of pain is a network of neuronal pathways and circuits responding to sensory (nociceptive) stimulation [13,16,17]. The neuromatrix theory of pain proposes that pain is a multidimensional experience produced by characteristic "neurosignature" patterns of nerve impulses generated by a widely distributed neural network in the brain [13,16]. These neurosignature patterns may be triggered by inputs such as tactile sensations. Tactile perception is an innate mechanism for human survival and represents our evolved and adaptive somatosensorial ability to apprehend information via haptics – the active touch for object recognition and perception by higher centers of the brain [18,19]. The somatosensory experience is determined by a set of channels and receptors sensitive to thermal, tactile, and mechanical stimuli shown to be critical to survival, balance control, and pain modulation[18-20]. The complex pain neuromatrix, an explanatory model of how pain is generated and therapeutically alleviated, suggests that pain originates and is experienced in specific clusters and patterns within neuronal impulses, which originate from a neural network dubbed “body-self neuromatrix” [13]. This challenges the theory that pain originates in a noxious stimulus causing tissue injury or damage, or the “Cartesian model of pain” [21,22].
The intricate neuronal signals associated with pain are measurable by the electroencephalogram (EEG) [17,23,24]. Decoding pain perception using EEG is an advancement that has a wide spectrum of physiological and pathophysiological ramifications. This reveals a spatio-temporal signature associated with pain, nociception, and hyperalgesia. EEG research has shown that haptic vibrotactile trigger technology (VTT) modulates brain centers that are associated with pain pathways [25]. In recent years, haptic skin- stimulation technology has been incorporated into several over- the-counter products with different routes of delivery that include patches, apparel (socks), braces, wrist bands, and compression sleeves, among others.
In this pilot HARMONI (Health Assessments: Reviewing, Measuring, and Observing Neuromatrix Interaction) study, we evaluate a non-invasive pain-relieving patch (FREEDOM Super Patch with VTT; Srysty Holding Co.,Toronto, Canada) that incorporates haptic-vibrotactile trigger technology (VTT). This minimal risk, observational study, evaluated this over-the- counter (OTC) non- pharmacological patch that is embedded with proprietary sensory patterns and incorporating VTT. The patch is designed to trigger neural pathways and circuits associated with the neuromatrix of pain and other cortical networks. This study included patients with mild/moderate/severe, and acute or chronic pain and evaluated their overall perceptions of pain treatment and associated symptoms with the use of the VTT pain patch. The Brief Pain Inventory short form (BPI) tool was used to assess patient- reported changes in pain severity and pain interference scores and change in the use of pain medications at 7- and 14-days following treatment. Data presented here are on active treatment. Future planned analyses will include a control and a crossover group of patients and explore differences between each group.
Methods
Study Design
This study was a prospective, Institutional Review Board-approved Observational Study aimed at evaluating patients’ experiences and/ or perceptions and patient response for those who have received a haptic vibrotactile trigger technology (VTT) embedded patch (FREEDOM Super Patch with VTT; Srysty Holding Co.,Toronto, Canada) or an inactive pain patch by their clinician. Preliminary study data presented here include only subjects who received active treatment.
Baseline Demographic and Clinical Characteristics of Patients A total of 148 patients (96 females, 52 males) at 3 US investigator sites were enrolled in the treatment arm of the study and completed the baseline, day 7, and day 14 surveys. Demographic results were similar for gender and age at the baseline survey for all groups of patients. The mean age at baseline was 52.9 years. The primary pain complaint for the patients was recorded at baseline for all groups. (Table 1). Myofascial/musculoskeletal pain was the most prominent pain complaint indicated by 54/148 (36.5%) of patients. Forty-seven (47; 31.8%) patients indicated that neuropathy/ radiculopathy and Arthritis was their primary pain complaint.
At baseline, of the 54 study participants who indicated myofascial/ musculoskeletal pain as their primary complaint, 59% noted that their hips and lower extremities was the most common location of pain (n=33), followed by 39% (n=21) of patients indicating that their neck, back, and shoulders was the area of their pain. Of the remaining 47 patients who indicated arthritis as their primary pain complaint, 81% noted their lower extremities (hip, knee, and foot) was the most common location of their pain (n=38). Almost 30% of patients reported having their pain for 3 months to one year (43/148) and over 62% reported having pain for more than one year (93/148). BPI scores indicated that patients receiving the patch embedded with the haptic vibrotactile trigger technology (VTT) were experiencing mild (10%; 15/148), moderate (29%; 43/148), or severe pain (61%; 90/148).
Pain management and symptoms were evaluated by patient answers to validated pain measurement and symptom scales (e.g., Brief Pain Inventory (BPI)) as well as additional survey questions regarding patient satisfaction, patient quality of life, and resumption of their normal activities. Evaluation of a Control Group (CG) of patients (given an inactive vehicle patch) and a crossover group of patients (CROSSG) who received the active patch after 14 days of being in the control group, will also be included in future analyses.
Patients who met the eligibility criteria and who were treated with the pain-relieving patch comprised the study’s treatment group (TG). For the treatment group, patient inclusion criteria were as follows: 1) ages 18 to 85 years, inclusive; 2) ability to provide written informed consent; 3) received the active VTT embedded study patch; and 4) had been diagnosed with a mild/ moderate/severe, acute or chronic pain condition. Patients who had had a history of use drug or alcohol abuse, patients who had an implantable pacemaker, defibrillator or other electrical devices, or patients who were pregnant, were ineligible to participate in the study.
Each site provided patients an identification number, and a confidential file containing the informed consent forms and patient identification numbers were kept and maintained in a secured cabinet only accessible to the principal investigator and authorized personnel. Patient survey responses were provided with no identifying patient information.
Patients could withdraw from this study at any time with the assurance of no unfavorable impact on their medical care. All diagnostic tests and treatment decisions were made at the discretion of clinicians, with no tests, treatments, or investigations performed as part of this study. Patients were provided the treatment at no cost and were not compensated for their participation in the study.
The study protocol was approved by ADVARRA institutional review board and was performed in full accordance with the rules of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the principles of the declaration of Helsinki and the international council of Harmonisation/GCP. All patients gave informed and written consent.
Topical Intervention
The active, non-invasive, 2 x 2 inch non-pharmacological patches are embedded with proprietary sensory pattern imprints and incorporate haptic vibrotactile trigger technology (VTT). The active patches contain no drug or energy source. There is an adhesive backing on one side of the active patch. Patients in the treatment group were instructed to wear one patch near the site of pain and replace the patch each day (SEE PICTURE 1). The non-active patches look similar to the active patches but do not incorporate the haptic vibrotactile trigger technology (VTT).

Study Procedures and Assessments
Following enrollment, patients were asked to complete surveys at baseline (day 0) and follow-up on days 7 and 14 of the study period. The surveys were comprised of questions to address and document the nature and location of the primary pain complaint of the patient, which included: 1) arthritis; 2) neuropathy or radiculopathy; or 3) myofascial or musculoskeletal pain. (Locations included neck, shoulders, back, hands, feet, hips, knees, and neck, among others). Study participants indicated only one pain complaint/location, which was the intended patch area for the active and non-active treatment arms.
Included in the survey was the Brief Pain Inventory (BPI), a validated pain assessment tool that is brief and simple to use in
both clinical and research settings. This tool assesses not only the severity of pain (0-10 numeric rating scale), but importantly the impact of pain on daily function in patients with cancer pain and other pain conditions [26,27].We also queried location of pain, pain medications, and amount of pain relief in the past 24 hours or the past week.
For the questions about pain severity, 0 is “no pain” and 10 is “pain as bad as you can imagine.” For the questions about pain interference with activities of daily living, 0 is “does not interfere” and 10 is “completely interferes.” Patient responses to questions regarding pain severity (4 questions) and pain interference (7 questions) were compiled to yield the overall score for pain severity and pain interference.
Patients were asked to indicate any other medications that they had been taking for pain relief at the time of the baseline, day 7, and day 14. Categories of medications that patients could choose included OTC pain medication agents, prescription anti-inflammatory medications, muscle relaxers, opioids, and anticonvulsants. Patients could indicate use of more than one type/ class of analgesic medication.
Study End Points
The primary endpoints included changes in patient Brief Pain Inventory (BPI) overall severity and interference scores among the treatment group for the primary pain complaint, as well as changes in the use of prescription and OTC medications. We also assessed patient satisfaction with patch treatment and any side effects reported by patients during the trial. Future analyses will compare the non-active control and crossover treatment groups with the outcomes reported here.
Statistical Analysis
For all variables, descriptive statistics were calculated, including frequencies and percent for categorical variables and means with standard deviation (SD) for continuous variables. The maximum sample size available was used for each statistical analysis.
Changes from baseline to day 7, and to day 14, in BPI mean pain severity and pain interference scores were analyzed using the paired t-test to identify any statistically significant differences within the treatment group.
Each survey collected the numbers and types of prescription and OTC oral/topical medications being used for pain relief; statistically significant differences in the use of these types of medications from baseline to day 14 were determined using the McNemar test and χ2 test for binomial paired and unpaired data respectively. Descriptive statistics were used to determine patient satisfaction with the pain-relieving patch within those treated. Descriptive statistics were also used to report any side effects experienced by patients.
A two-tailed alpha was set to 0.05 for all statistical comparisons. SPSS v. 27 was used for all analyses.
Results
Treatment Group
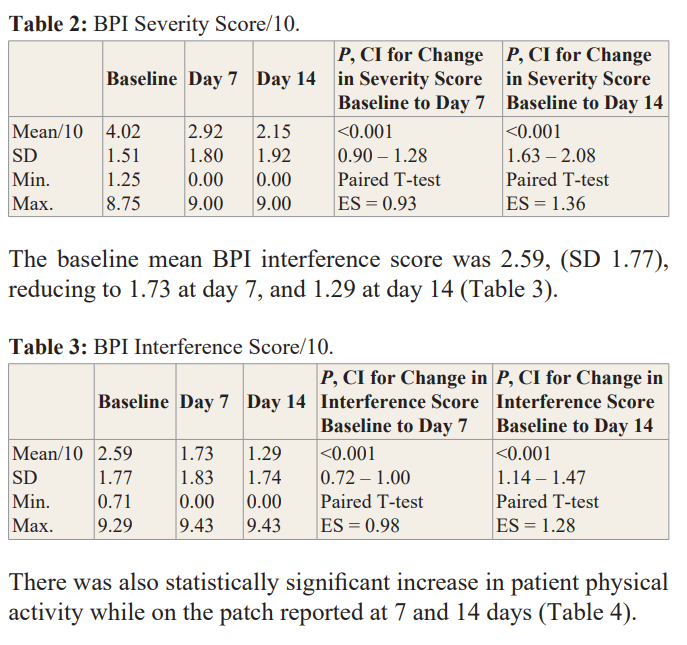
Treatment group paired data were collected; only patients that completed 14 days of treatment were included in the analysis. Over 14 days, mean BPI Severity score decreased 47% (4.02 to 2.15/10; p< .001) and mean BPI Interference score decreased 50% (2.59 to 1.29/10; p< .001). After 14 days, 82% of patients reported “less” or “a lot less” usage of oral medications. 75% of patients were satisfied with the treatment and of those, 83% were very/extremely satisfied with the patch. Results also showed statistically significant and positive outcomes (p<.0.001) in all measured Quality of Life (QoL) components with improvements in general activity, mood, relations with other people, sleep, normal work, walking ability, and enjoyment of life. Out of 148 patients, there was only one reported adverse event (swelling) deemed as non-serious by the treating clinician.
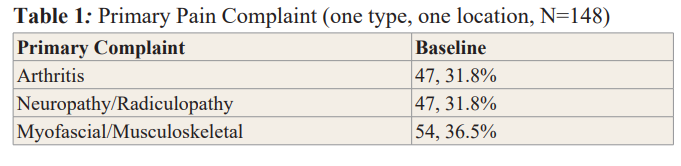
Changes in BPI Severity and Interference Scores
The mean BPI pain severity score at baseline was 4.2 (SD 1.51), reducing to 2.92 at day 7, and 2.15 at day 14 (Table 2).
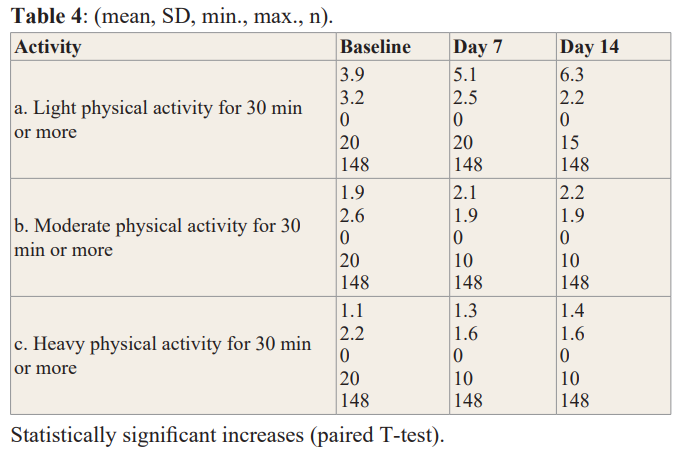
Light Physical Activity: Baseline to Day 7, Baseline to Day 14,and Day 7 to Day 14: each statistically significant at p<0.001.
Moderate Physical Activity: Baseline to Day 14 (p=0.007). Day 7 to Day 14 (p=0.026).
Heavy Physical Activity: Baseline to Day 7 (p=0.014). Baseline to Day 14 (p=0.002).
Changes in Self-Perceived Pain Relief from Medications
One of the BPI questions (not part of the pain severity or interference scores) asks the patient how much pain relief (in increments of 10% from 0% = no relief to 100% = complete relief) they have experienced from treatments or medications within the last 24 hours. At baseline, patients reported a mean of 27.6% pain relief from current treatment or medications; by day 7 they reported 61% pain relief, and by day 14 they reported 72% pain relief. The change in mean percent relief from baseline to day 7 was statistically significant (95% CI, 29.2 to 37.9 p < .001) and was also significant from baseline to day 14 (95% CI, 39.2 to 49.4,p < .001).
Changes from Baseline to Day 7 and Baseline to Day 14 in the Use of Concurrent Pain Medications
Patients indicated their utilization of pharmacological treatments for pain at baseline, 7 days, and 14 days. Treatments included OTC agents, prescription anti-inflammatory medications, opioids, anticonvulsants, or muscle relaxants, or a combination of those four classes. At Baseline, there were 56% of patients (83/148) taking an OTC product for their pain, 53% of patients (79/148) taking a prescription anti-inflammatory, 16% (24/148) taking a muscle relaxant, and 2% (3/148) taking an opioid or anticonvulsant.
There was a decrease in the number of patients using one or more OTC pain medications from Baseline to day 7 and from Baseline to day 14. Approximately 44% of patients reported using Ibuprofen, Naproxen, and/or Acetaminophen at Baseline (65/148). As far as prescription anti-inflammatory medication, naproxen was reported most often 28/148 (19%). At day 14, only 11 patients reported that they were still using a prescription anti-inflammatory. This is reduction from 79 at Baseline. This is a statistically significant decrease of p <.001. Also noted was a statistically significant decrease in the number of patients using one or more muscle relaxers from Baseline to Day 7 (24 to 2 patients), and Baseline to Day 14 (24 to 1 patient), p<0.001 for each. Although a minority of patients reported using opioids or anticonvulsants at baseline (3, 2%), all but 1 patient discontinued their prescription opioids and anticonvulsants by day 7 which persisted through day 14. Separate from indicating use of specific medications for pain, patients were asked how their use of oral pain medications had changed (scale: 1 = A lot more, 2 = More, 3 = No change, 4 = Less, 5 = A lot less). At day 7, 78% reported “less” or “a lot less.” At day 14, 82% reported “less” or “a lot less.”
Satisfaction with Use of the Pain Patch
Subjects were queried on specific satisfaction rating aspects regarding use of the pain patch (scale: 1 = Strongly disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly agree). At day 14, the mean ratings were 4.6 for each of “easy to apply” and “convenient,” and 4.2 for each of “preferred over pills/oral medication” and “preferred over other pain-relieving treatments.” At day 14, overall satisfaction was 4.1 out of 5 (scale: 1 = not at all, 2 = Not very, 3 = Somewhat, 4 = Very, 5 = Extremely).
Use of the Patch
At day 14, 134/148 (91%) of patients reported that they kept the patch on ‘almost all of the time.’ Of the remaining 14 patients, 6 patients reported that they used the patch ‘until the pain was gone, then again when the pain came back.’ At the first follow-up data collection point at day 7, 82/148 (55%) of patients reported that they felt pain relief in less than 20 minutes after applying the patch. 35% of patients (52/148) reported that it took longer than 20 minutes to feel pain relief. At day 14, 109/148 (74%) of patients reported that they felt pain relief in less than 20 minutes after application and 23/148 (16%) of patients reported pain relief after 20 minutes.
Duration of Pain Relief
At day 14, patients were asked how long it took for the pain to return once they removed the patch. Approximately 10% of patients reported that their pain did not return after they removed the patch; 44% of patients (65/148) reported that it took longer than one day for the pain to return after patch removal, and 31/148 (21%) of patients reported that pain was still absent after 2 hours of removing the patch.
Safety
Patients reported no adverse skin reactions or serious adverse events while being treated with the pain relief patch.
Discussion
Here we report results of this HARMONI study, a prospective, non-randomized observational study evaluating the safety and analgesic efficacy of the FREEDOM Super Patch with VTT in patients presenting with mild, moderate and even severe musculoskeletal, arthritic and neurological pain. This analysis showed improvements in BPI pain severity and pain interference scores and use of concurrent pain medications from baseline to day 7, and to day 14.
Research surrounding haptic vibrotactile trigger technology (VTT) has shown that there is a change in EEG patterns in those patients exposed to VTT [25]. Over the past several years, researchers have developed an understanding of the Neuromatrix Theory of Pain (NTP) through a broad base of imaging studies and related theories of how different brain regions interact and sense pain [13-15].
Chronic pain perception appears to involve multiple neural pathways in addition to those associated with acute pain [16,17]. The networks involved in the perception of painful sensations, as well as their communication and coordination between the CNS and PNS, are broadly referred to as the “neuromatrix” -- the basis for the NTP [13].
The NTP, first proposed by Ronald Melzack, hypothesized that networks of neurons communicating in “large loops”, or through continuous cyclical processing, connect specific regions of the brain with the PNS during sensory processing [13]. He envisioned 3 distinct looping pathways: 1) a traditional sensory pathway with neural projections routed through the thalamus, 2) one that follows a path through the brainstem and parts of the limbic system, and 3) one associated with pathways that are routed through different Brodmann Areas (BA), particularly the somatosensory cortex. These loops were meant to explain the cognitive, emotional, and motor modalities through which humans experience sensations, particularly pain [13,14].
The EEG mapping of the pain neuromatrix is corroborated with neuroimaging techniques such as functional analysis using magnetic resonance imaging (fMRI) in many experimental paradigms. The sensory patterns within the patches are in close symmetry between known EEG patterns and their role in modulating EEG and neuronal circuits within higher brain centers. Perceptual, motor, and autonomic responses occupy distinct patterns of the EEG conundrum of pain. It has been shown that pain-related activation of the anterior and posterior cingulate cortices (ACC and PCC, respectively) can lead to identification of various somatosensory circuits associated with proximal and distal sites of the median nerves. This is corroborated by the observations that primary and secondary somatosensory cortices, insular cortex, ACC, prefrontal cortex (PFC), and thalamus are activated centers within the neuromatrix [14].
The brain centers targeted by VTT have been shown to be responsive to external stimuli that incorporate the VTT technology and have produced positive outcomes in balance and stability measurements [28].
There remains an unmet need for alternative treatment options for patients with pain. Potential life-threatening adverse effects have been shown with NSAIDS, acetaminophen, opioids and adjuvant analgesics. Novel, non-pharmacologic and non-invasive therapies fulfill an unmet need for additional safe and effective treatment strategies and options for patients experiencing pain [29-34].
Limitations
This was a nonrandomized, observational study based on a sample of patients attending diverse clinical settings for the treatment of arthritic, neurological, and musculoskeletal pain who consented to participate in this study. This analysis reported on a group of 148 patients who were treated with the VTT embedded study patch.
The data of those patients who did not complete the follow up surveys after baseline, or patients who indicated that they did not use the patch after the baseline visit were removed from evaluation. Due to patients having different primary pain complaints and specific location of their pain, overall generalization and consistency of results may be impacted due to the different location of pain, the amount of time the patient utilized the patch, and subjective self-reporting by the patient. We have attempted to accurately evaluate and provide the most detailed reporting of the data while considering these limitations. Inclusion of control group and crossover group data in future analyses will assist in confirming the validity of these results due to the nonrandomized nature of this clinical trial.
Conclusion
Study results indicate that this non-pharmacologic, non-invasive, haptic vibrotactile trigger technology (VTT) embedded topical patch reduces pain severity and interference scores and may reduce the use of concurrent medications, including prescribed anti-inflammatory and other oral medication for adult patients with arthritic, neuropathic, and musculoskeletal pain. Results reported here from this IRB-approved observational study suggests that the non-pharmacological topical pain patch embedded with VTT technology has incredible potential to be added to the current arsenal of noninvasive and nonpharmacological pain therapies. Further evaluation, including data from control and crossover groups are forthcoming and should support the use of this OTC pain patch as a first-line non-pharmacological treatment option as part of a multimodal treatment approach.
Acknowledgments
This IRB-approved study administered by Clarity Science LLC was funded by Srysty Holding Co., the distributors of the FREEDOM Super Patch with VTT®.
Disclosure
Jeffrey Gudin MD has received compensation from Clarity Science LLC for his role as principal investigator and for providing protocol- required services for the study. Peter L Hurwitz is President of Clarity Science LLC. Derek T Dietze received compensation for study statistical analyses. Galen Dhaliwal did not receive any compensation for assistance with manuscript development. The authors report no other disclosures.
References
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician. 2018; 64: 832-840.
- Jones J, Rutledge DN, Jones KD, et Self-assessed physical function levels of women with fibromyalgia: a national survey. Womens Health Issues. 2008; 18: 406-412.
- Dueñas M, Ojeda B, Salazar A, et A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016; 9: 457-467.
- Dunsky The Effect of Balance and Coordination Exercises on Quality of Life in Older Adults: A Mini-Review. Front Aging Neurosci. 2019; 11: 318.
- Cuomo A, Bimonte S, Forte CA, et Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. J Pain Res. 2019; 12: 711-714.
- Sharon L Kolasinski, Tuhina Neogi, Marc C Hochberg, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken). 2020; 72: 149-162.
- Gao YJ, Ji Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010; 7: 482-493.
- Cuomo A, Bimonte S, Forte CA, et Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. J Pain Res. 2019; 12: 711-714.
- Gudin JA, Dietze DT, Hurwitz Using Nanotechnology to Improve Pain and Function with a Novel, Drug-Free, Topical Pain-Relief Patch: An Interim Analysis. Anesth Pain Res. 2020; 4: 1-10.
- Gudin JA, Dietze DT, Hurwitz PL. Improvement of Pain and Function After Use of a Topical Pain Relieving Patch: Results of the RELIEF Study. J Pain Res. 2020; 13: 1557-1568.
- Argoff Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc. 2013; 88: 195-205.
- Moayedi M, Davis Theories of pain: from specificity togate control. J Neurophysiol. 2013; 109: 5-12.
- Melzack Pain and the neuromatrix in the brain. J Dent Educ. 2001; 65: 1378-1382.
- Derbyshire SWG. Exploring the pain “neuromatrix.” Curr Rev Pain. 2000; 4: 467-477.
- Mouraux A, Diukova A, Lee MC, et al. A multisensory investigation of the functional significance of the “pain matrix.” Neuroimage. 2011; 54: 2237-2249.
- Weiss Plasticity and cortical reorganization associated with pain. Z Psychol. 2016; 224: 71-79.
- Diers M, Koeppe C, Diesch E, et al. Central processing of acute muscle pain in chronic low back pain patients: an EEG mapping study. J Clin Neurophysiol. 2007; 24: 76-83.
- Fernandes AM, Albuquerque PB. Tactual perception: A review of experimental variables and procedures. Cogn Process. 2012; 13: 285-301.
- Reed CL, Ziat Haptic perception: From the skin to the brain. In Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier. 2018.
- Büchel D, Lehmann T, Ullrich S, et Stance leg and surface stability modulate cortical activity during human single leg stance. Exp Brain Res. 2021; 239: 1193-1202.
- Trachsel LA, Munakomi S, Cascella M. Pain Theory. Stat Pearls. Treasure Island (FL): Stat Pearls. 2022.
- Wolnei Caumo, Maria Beatriz Cardoso Perioperative anxiety: psychobiology and effects in postoperative recovery. The Pain Clinic. 2003; 15: 87-101.
- Hsueh J, Chen JJ, Shaw F. Distinct somatic discrimination reflected by laser-evoked potentials using scalp EEG Journal of Medical and Biological Engineering. 2016; 36: 460-469.
- LenoirD,WillaertW,CoppietersI,etal.Electroencephalography during nociceptive stimulation in chronic pain patients: a systematic review. Pain Medicine. 2020; 21: 3413-3427.
- Dhaliwal BS, Haddad J, Debrincat M, et al. Changes in Electroencephalogram (EEG) After Foot Stimulation with Embedded Haptic Vibrotactile Trigger Technology: Neuromatrix and Pain Modulation Considerations. Anesth Pain Res. 2022; 6: 1-11.
- Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT J Pain. 2008; 9: 105-121.
- Mendoza TR, Chen C, Brugger A, et al. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain. 2004;20: 357-362.
- Haddad J, Dhaliwal BS, Dhaliwal MS, et Improvement in Balance and Stability Using a Novel Sensory Application: Haptic Vibrotactile Trigger Technology. Int J Res Phys Med Rehabil. 2022; 1: 1-7.
- Farkouh ME, Greenberg An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 2009; 103: 1227-1237.
- Harirforoosh S, Jamali Renal adverse effects of nonsteroidal anti- inflammatory drugs. Expert Opin Drug Saf. 2009; 8: 669-681.
- John R, Herzenberg Renal toxicity of therapeutic drugs.J Clin Pathol. 2009; 62: 505-515.
- Lazzaroni M, Porro GB. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009; 69: 51-69.
- Scarpignato C, Hunt RH. Nonsteroidal anti-inflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010; 39: 433-464.
- Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011; 342: 7086.