Life-Threatening Amniotic Fluid Emboli Requiring Massive Blood Transfusion and Veno-Arterial Extracorporeal Membrane Oxygenation Therapy: Case Report and Review of the Literature
Author'(s):Dichtwald Sara MD1*, Khashan Ahmed MD1, Meyer Avraham MD1, Gorfil Dan. M MD2, Ifrach Nisim MD1and Drori Idan MD1
1Department of Anesthesiology, Intensive Care and Pain Medicine,Meir Medical Center, Kfar Saba, Israel. Affiliated with the Sackler School of Medicine, Tel Aviv University, Israel.
2Department of Cardiothoracic surgery, Rabin Medical Center,Petah-Tikva, Israel. Affiliated with the Sackler School of Medicine,Tel Aviv University, Israel.
*Correspondence:
Sara Dichtwald, MD, Department of Anesthesiology, Intensive Care and Pain Medicine, Meir Medical Center, 59 Tchernichovsky St. Kfar Saba 4428164 Israel, Tel: 972-9-7472133
Received: 01 Nov 2022; Accepted: 25 Nov 2022; Published: 30 Nov 2022
Citation: Sara D, Ahmed K, Avraham M, et al. Life-Threatening Amniotic Fluid Emboli Requiring Massive Blood Transfusion and Veno-Arterial Extracorporeal Membrane Oxygenation Therapy: Case Report and Review of the Literature. Anesth Pain Res. 2022; 6(2): 1-5.
Abstract
Background: Amniotic fluid embolism (AFE) is a rare and disastrous condition that occurs during labor or soon after delivery. Its course is abrupt and rapidly progressive and may result in cardiorespiratory collapse, profound hemorrhage and multi organ failure. Mortality and morbidity are high, with many survivors suffering major neurologic sequelae. Disseminated intravascular coagulation (DIC), severe coagulopathy and hemorrhage may pose a risk for extracorporeal membrane oxygenation (ECMO) therapy due to the need of full anticoagulation treatment.
Case Report: A 41 year old healthy parturient who developed fulminant AFE during cesarean delivery, accompanied by cardiac arrest, severe acute respiratory distress syndrome and DIC with profound obstetric hemorrhage. She was placed on veno-arterial ECMO as a rescue therapy and required more than 850 blood products. She had concomitant septic shock with E-coli bacteremia due to chroioamnionitis. Despite prolonged and complicated ICU course, including recurrent hemorrhage, multiple surgeries and sepsis, she survived to hospital discharge without neurologic sequelae.
Conclusion: ECMO therapy may be considered as a rescue therapy in AFE cases, refractory to standard cardiorespiratory support, despite the increased risk for hemorrhage under full anticoagulation therapy.
Interdisciplinary coordination is mandatory for a good outcome.
Keywords
Introduction
Amniotic fluid embolism (AFE) is a rare and disastrous condition that occurs during labor or delivery. It is a diagnosis of exclusion, and should be suspected in pregnant or recently post-partum women with sudden cardiovascular compromise. Clinical findings include hypoxemia, seizures, sudden loss of consciousness, shock and cardiac arrest. Profound bleeding and disseminated intravascular coagulation soon appear. Differential diagnosis includes peripartum hemorrhage, severe pre-eclampsia or eclampsia, pulmonary embolism, sepsis or septic shock and peripartum cardiomyopathy.
Diagnostic criteria were proposed by the society for maternal-fetal medicine (SMFM), including sudden onset of cardiorespiratory arrest or hypotension, with evidence of respiratory compromise, and disseminated intravascular coagulation (DIC), as documented by platelet count below 100,000/ml, prolonged prothrombin time and fibrinogen level below 200 mg/dl, along with clinical onset during labor or within 30 minutes of placental delivery, with absence of maternal fever during labor [1]. AFE incidence ranges from 1.9 to 6.1 cases per 100,000 deliveries [2], but it may be underestimated due to misdiagnosis.
The pathogenesis of AFE is unclear. It is assumed that during delivery, amniotic fluid and fetal cells enter the maternal circulation, leading to profound activation of the immune system, resulting in extensive release of cytokines, pro-coagulant factors and inflammatory mediators, causing "cytokine storm". Another assumption is that bacterial antigens may also enter the maternal blood stream during this process, further aggravating the immune response in a "toxic shock-like" manner [3]. The systemic inflammation is responsible for pulmonary vasoconstriction, resulting in acute pulmonary hypertension, right ventricular failure and shock, capillary leak with pulmonary edema and hypoxemia, and disseminated intravascular coagulation [3,4]. Hemorrhage, ischemia and multi-organ failure ensue.
Risk factors are unclear either, but are potentially assumed to include older maternal age, cesarean section, instrumental vaginal delivery (forceps or vacuum-assisted delivery), placenta abruption and pre- eclampsia [5,6]. Common clinical symptoms include abrupt and catastrophic onset, with sudden hypoxemia and cardiorespiratory collapse, pulmonary edema, hemorrhage, chills, nausea and vomiting, confusion, seizures and coma [7]. Laboratory findings include elevated D-dimers with low fibrinogen, thrombocytopenia, anemia, elevated white blood cell count and metabolic acidosis [7]. Chest x-ray may reveal bilateral lung infiltrates suggesting pulmonary edema. Echocardiography usually reveals increased pulmonary artery pressure with decreased contractility of the right ventricle, followed by left ventricular failure.
There is no specific treatment for AFE. Supportive treatment includes delivery of the fetus, cardiopulmonary resuscitation, hemodynamic support with fluids and vasopressors, blood products in case of severe hemorrhage and DIC, and intubation with ventilator support. Inotropic support may be required in case of left ventricular failure. There are some anecdotal reports using intralipid [8,9] but its efficacy is unclear yet. Extra corporeal membrane oxygenation (ECMO) is a rescue therapy in refractory cases, but should not be used routinely, since anticoagulation is required and the risk of hemorrhage is increased, due to AFE- related DIC. There are several case reports suggesting successful use of ECMO in extreme AFE cases that did not respond to standard respiratory and hemodynamic support [10-15]. Prognosis is poor, with maternal mortality ranging from 10-90% [7] in older reports, to 20-50% in more recent studies [16]. About 85% of the survivors will suffer significant neurologic injury [7]. No case of recurrent AFE has been reported among survivors, so the risk of recurrent AFE is unknown. Successful pregnancies and deliveries following AFE have been documented [17].
Here we present a case of a young woman who developed both amniotic fluid embolism and E-coli bacteremia during cesarean delivery, with DIC and profound bleeding, requiring massive blood transfusion and ECMO therapy.
Case Presentation
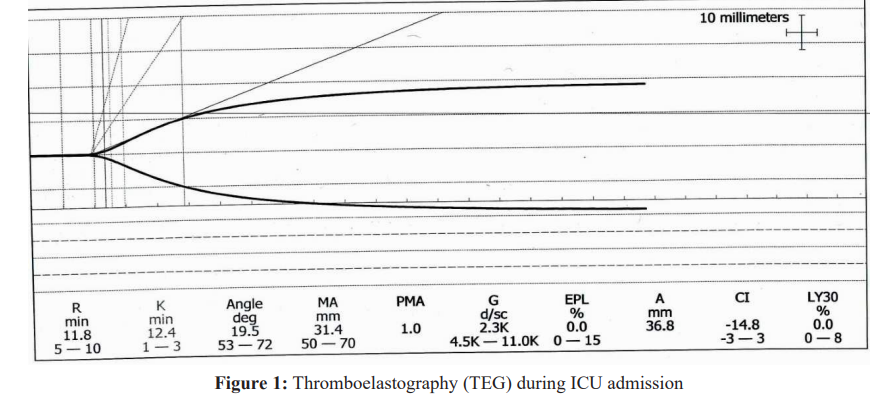
S.M, a healthy 41-year-old woman, 38+5 weeks pregnant in her second pregnancy was admitted for cesarean section due to maternal fever, suspected chroioamnionitis and fetal tachycardia. On admission she was stable, had spinal anesthesia and the surgery began without any adverse events. After extraction of the fetus, she complained of sudden shortness of breath, became agitated and bradycardic, then became comatose and went into cardiac arrest. Asystole was observed on the monitor. The surgeons were noted of the situation and immediate chest compression followed by intubation and mechanical ventilation ensued. She received epinephrine. After three rounds of cardiopulmonary resuscitation return of spontaneous circulation was observed. Echocardiography revealed dilated and hypokinetic right ventricle. She started to bleed profoundly from the uterus and then from the endotracheal tube, nasogastric tube and venipuncture sites. DIC was diagnosed. Due to uterine atony, uterine massage was performed by the surgeon and uterine constrictors were given by the anesthesiologist. She received packed blood cells, fresh frozen plasma, platelets and cryoprecipitate, along with tranexamic acid and recombinant factor VIIa (NovoSeven® RT). She was in profound shock and received high doses of epinephrine and norepinephrine, with severe lactic acidosis, coagulopathy- as observed in Thromboelastography (Figure 1) and hypoxemia. Since hemorrhage was assumed to have ceased, she was transferred to the intensive care unit (ICU).
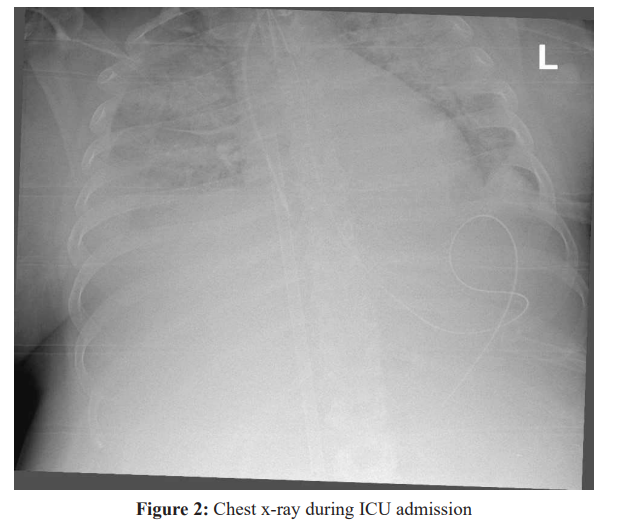
Soon after ICU admission, she had another hemodynamic collapse and asystole. Cardiopulmonary resuscitation was again initiated, and due to severe hemorrhage from the surgical wound and drains she was rushed back to the operating room in hemorrhagic shock. Massive uterine bleeding was observed. She received more blood products and coagulation factors, but the bleeding could not be controlled and emergency hysterectomy was performed with packing of the pelvis and abdomen. She was transferred back to the ICU in critical condition, severe hypoxemia with PO2/FiO2 ratio of less than 80, bilateral dense infiltrates on chest x-ray (Figure 2), cardiogenic shock with both severely hypokinetic ventricles as observed in echocardiography, very high doses of inotropic and vasopressor support, severe DIC and lactic acidosis. Due to refractory cardiorespiratory collapse, therapy with veno- arterial ECMO was initiated, in spite of coagulopathy, as a last resort measure. Cannulation of the right femoral artery and vein was performed along with distal perfusion.
She continued to receive multiple blood products due to profound bleeding and DIC. Empiric antibiotic therapy with Ampicillin, Gentamycin and Metronidazole was begun. During the first 48 hours she was in grave condition, still requiring high inotropic and vasopressor support despite ECMO therapy, had DIC and bleeding from multiple sites including cannulation areas, and severe lactic
acidosis with lactate level exceeding the maximal detectable level. Echocardiography still revealed severe right and left ventricular dysfunction with moderate to severe pulmonary hypertension. Blood cultures taken during the cesarean section were positive for E-coli, as were placental cultures. We continued therapy with Cefuroxime and Metronidazole. She had acute kidney injury and had no urine output, and therefore was placed on continuous veno- venous hemodialofiltration (CVVHDF).
After 48 hours de-packing was performed bedside, again with profound bleeding of more than 3 liters from the pelvis. She received massive blood transfusion including two separate doses of recombinant factor VIIa (NovoSeven® RT) and packing was again performed. After another 48 hours, after certain stabilization was achieved, she underwent computed tomography demonstrating active bleeding from the rectus muscles, bilaterally, from the mesocolon and from multiple sites in the pelvis. Computed tomography of the brain revealed small intracerebral bleeding from the falx cerebri.
Angio-embolization of both inferior epigastric arteries was performed, and she was transferred to the operation room, had de-packing with resumption of profuse hemorrhage. Tight sutures were densely placed on the rectus muscles in order to control the bleeding, and the pelvis was packed again.
48 hours later, she had another laparotomy and de-packing. No active bleeding was observed.
Her oxygenation and cardiac function improved, coagulation profile normalized and she was weaned of ECMO after 8 days. She underwent tracheostomy and gradually regained full consciousness. She remained with severe kidney injury and anuria, and hemodialysis was continued. She developed severe intra-abdominal infection with septic shock, with bacteremia of resistant Klebsiella Pneumonia and had multiple laparotomies with abdominal lavage due to multiple abscesses. The gastrointestinal tract was not involved in the infectious process. The abdomen was left open with ABTHERATM vacuum dressing, and was gradually closed, eventually with a skin graft.
During her 86 days in the ICU, she received a total of 867 blood products, including 183 units of packed red blood cells, 124 units of fresh frozen plasma, 220 units of cryoprecipitate and 340 units of platelets. As a result, she had severe hyperbilirubinemia with total bilirubin level exceeding 20 mg/dl, which gradually decreased to normal levels. Renal function improved, even though she had low residual kidney function with creatinine clearance of 15-20 cc/min. However, she did not require further dialysis therapy. She was gradually weaned of the ventilator and successful de cannulation was performed. Echocardiography showed improvement in left and right ventricular function, with ejection fraction of 50%. After 105 days in the ICU, she was discharged to rehabilitation awake and cooperative, without major neurologic deficits, breathing spontaneously after de-cannulation, in a stable condition. Her newborn healthy daughter was discharged from the nursery soon after her birth.
Discussion
AFE is a catastrophic obstetric complication, with high morbidity and mortality, and abrupt and rapidly progressive course. It may be difficult to diagnose in real-time, with a broad differential diagnosis. Even though the diagnosis of AFE in the presented case was made promptly, since the patient presented with all the classic typical symptoms, her condition was more complicated than perceived at first, since not only she had fulminant AFE with cardiorespiratory collapse and multi-organ failure, but also had septic shock due to chroioamnionitis and E coli bacteremia. Since in real time it may be hard to diagnose AFE, especially in atypical cases, it may be reasonable to give empirical treatment aimed at all possible causes in the rapidly deteriorating parturient. Basic and supportive care with cardiopulmonary resuscitation, intubation, fluids, inotropic and vasopressor support (guided by echocardiography) should be promptly initiated regardless of the primary etiology.
It seems reasonable to initiate empiric antibiotic therapy to treat possible sepsis (in addition or as an alternative differential diagnosis for AFE), since concomitant AFE and sepsis is a possibility, and sepsis may present with similar clinical manifestations to AFE.
Local anesthetics toxicity is another option in the rapidly deteriorating parturient who received neuroaxial analgesia for labor or surgery. High or total spinal anesthesia or accidental intravenous injection of local anesthetics may cause similar clinical symptoms, including coma, seizures and cardiorespiratory collapse. It may be reasonable to administer a bolus of intralipid emulsion, which may be life-saving in case of local anesthetics toxicity, and will be harmless in case of other etiologies. In fact, there are case reports of intralipid administration as a targeted treatment for AFE, even though its efficacy in those circumstances is unclear [8,9].
Anaphylactic shock is another possible etiology for cardiorespiratory collapse in the parturient. In the context of cesarean delivery, it may be caused by latex allergy, antibiotic administration or anesthetic agents (such as muscle relaxants during cesarean delivery under general anesthesia). Respiratory and cardiovascular support is the mainstay of therapy, along with administration of epinephrine, which is administered in the context of cardiopulmonary resuscitation anyway. It is interesting to note that serum tryptase level, typically elevated in anaphylactic shock, may also be elevated in AFE, indicating activation of mast cells as an integral part of the severe systemic inflammatory response [18].
Control of hemorrhage and correction of coagulation factors should be initiated as soon as possible. Thromboelastography (TEG) may be used as a point-of-care test to guide transfusion of blood products later in the course of disease [19]. Fibrinogen concentrates and tranexamic acid should be considered early during the course of DIC [20]. The administration of recombinant factor VIIa is controversial, as its use in peripartum hemorrhage has been associated with severe thrombotic morbidity and mortality [21]. However, in extreme cases when massive coagulation factor replacement is insufficient to improve hemostasis and stop the hemorrhage, its use should be considered [20].
Hysterectomy should be considered in the case of profound and continuous hemorrhage despite massive coagulation factor replacement and maximal surgical efforts. After the cesarean section, it seemed at first that the bleeding has stopped. Perhaps we should have waited with the patient longer in the operation room after the cesarean section, to rule out ongoing hemorrhage and perhaps to perform hysterectomy earlier, and not after the second cardiopulmonary collapse during ICU admission. In spite of the obvious traumatic and irreversible consequences of such a procedure, it may be lifesaving and hesitation to do so, although understandable, may be catastrophic. Moreover, in the case of fulminant AFE with severe cardiorespiratory collapse ECMO is a feasible option. Since veno-arterial ECMO requires full anticoagulation therapy, uncontrolled hemorrhage may prevent administration of this life-saving therapy. Continuous hemorrhage from the uterus, under full anticoagulation therapy, will be disastrous. Hysterectomy may be a definite solution in this context, and should be sought preemptively if ECMO is considered under those grave circumstances.
Coordinated interdisciplinary intervention and treatment are essential in order to improve prognosis in AFE. Obstetricians, anesthesiologists, pediatricians, intensivists, and experts in general surgery, vascular surgery and cardiothoracic surgery, ECMO technicians, infectious disease specialists, cardiologists, hematologists and ICU nurses are all essential to coordinate and participate in the complicated process. DIC, severe coagulopathy and massive bleeding in extreme AFE cases mandates meticulous coordination with hematologists and blood bank personnel.
Conclusion
ECMO therapy may be considered as a rescue therapy in AFE cases, refractory to standard cardiorespiratory support. DIC and coagulopathy may pose a challenge due to the need of full anticoagulation therapy, but in extreme cases, it may be lifesaving despite the risks. Interdisciplinary coordination is mandatory for a good outcome.
References
1.Clark SL, Romero R, Dildy GA, et al. Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am J Obstet Gynecol. 2016; 215: 408-412.
2.Knight M, Berg C, Brocklehurst P, et al. Amniotic fluid embolism incidence, risk factors and outcomes: a review and recommendations. BMC Pregnancy Childbirth. 2012; 12: 7.
3.Sultan P, Seligman K, Carvalho B. Amniotic fluid embolism: update and review. Curr Opin Anaesthesiol. 2016; 29: 288- 296.
4.Luis D Pacheco, George Saade, Gary DV Hankins, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol 2016; 215: 16-24.
5.Lawson HW, Atrash HK, Franks AL. Fatal pulmonary embolism during legal induced abortion in the United States from 1972 to 1985. Am J Obstet Gynecol. 1990; 162: 986- 990.
6.Hasaart TH, Essed GG. Amniotic fluid embolism after transabdominal amniocentesis. Eur J Obstet Gynecol Reprod Biol. 1983; 16: 25-30.
7.Clark SL, Hankins GD, Dudley DA, et al. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995; 172: 1158-1167.
8.Lynch W, McAllister RK, Lay JF Jr, et al. Lipid Emulsion Rescue of Amniotic Fluid Embolism-Induced Cardiac Arrest: A Case Report. AA Case Rep. 2017; 8: 64-66.
9.Igor G, Vladimir K, Michael D, et al. Intralipid Rescue of Amniotic Fluid Embolism: from Theory to Existence. J Health Sci Dev. 2019; 2: 1.
10.Stanten RD, Iverson LI, Daugharty TM, et al. Amniotic fluid embolism causing catastrophic pulmonary vasoconstriction: diagnosis by transesophageal echocardiogram and treatment by cardiopulmonary bypass. Obstet Gynecol. 2003; 102: 496-498.
11.Hsieh YY, Chang CC, Li PC, et al. Successful application of extracorporeal membrane oxygenation and intra-aortic balloon counterpulsation as lifesaving therapy for a patient with amniotic fluid embolism. Am J Obstet Gynecol. 2000; 183: 496-497.
12.Ecker JL, Solt K, Fitzsimons MG, et al. Case records of the Massachusetts General Hospital. Case 40-2012. A 43-year-old woman with cardiorespiratory arrest after a cesarean section. N Engl J Med. 2012; 367: 2528-2536.
13.Firstenberg MS, AbelE, Blais D, et al. Temporary extracorporeal circulatory support and pulmonary embolectomy for catastrophic amniotic fluid embolism. Heart Surg Forum. 2011; 14: 157-159.
14.Lee PH, Shulman MS, Vellayappan U, et al. Surgical treatment of an amniotic fluid embolism with cardiopulmonary collapse. Ann Thorac Surg. 2010; 90: 1694-1696.
15.Viau-Lapointe J, Filewod N. Extracorporeal Therapies for Amniotic Fluid Embolism. Obstet Gynecol. 2019; 134: 989- 994.
16.Abenhaim HA, Azoulay L, Kramer MS, et al. Incidence and risk factors of amniotic fluid embolisms: a population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008; 199: 49.
17.Caeiro AFC, Ramilo IDTM, Santos AP, et al. Amniotic Fluid Embolism. Is a New Pregnancy Possible? Case Report. Rev Bras Ginecol Obstet. 2017; 39: 369-372.
18.Fineschi V, Gambassi R, Gherardi M, et al. The diagnosis of amniotic fluid embolism: an immunohistochemical study for the quantification of pulmonary mast cell tryptase. Int J Legal Med. 1998; 111: 238-243.
19.Loughran JA, Kitchen TL, Sindhakar S, et al. Rotational thromboelastometry (ROTEM®)-guided diagnosis and management of amniotic fluid embolism. Int J Obstet Anesth. 2019; 38: 127-130.
20.Rath HR, Hofer S, Sinicina I. Amniotic fluid embolism: an interdisciplinary challenge. Dtsch Arztebl Int. 2014; 111: 126- 132.
21.Lockwood CJ, Bach R, Guha A, et al. Amniotic fluid contains tissue factor, a potent initiator of coagulation. Am J Obstet Gynecol. 1991; 165: 1335-1341.