Mechanistic Similarity of Immuno-modulatory and Anti-viral Effects of Chloroquine and Quercetin (The Naturally Occurring Flavonoid)
Author'(s): Anwar E1, Soliman M2, Darwish S3, Lotfy H4 and Tolba M5
1 PhD in Clinical Pharmacology. lecturer in Clinical Pharmacology department, Alexandria Faculty of Medicine, Egypt.
2Associate Professor of Pharmacology, University of Massachusetts, USA, Professor in Clinical Pharmacology, Alexandria Faculty of Medicine, Egypt.
3 Professor in Clinical Pharmacology, Alexandria Faculty of Medicine, Egypt.
4 Assistant Professor of Medical Microbiology and Immunology,Sulaiman Al Rajhi University, KSA.
5Assistant Professor, Department of Parasitology, Medical Research Institute, Alexandria University, Egypt.
*Correspondence:
Dr. Hany Lotfy El-Sawah. Assistant Professor of Medical Microbiology and Immunology, Sulaiman Al Rajhi University, KSA.
Received: 14 July 2020; Accepted: 01 August 2020
Citation: Anwar E, Soliman M, Darwish S, et al. Mechanistic Similarity of Immuno-modulatory and Anti-viral Effects of Chloroquine and Quercetin (The Naturally Occurring Flavonoid). Clin Immunol Res. 2020; 4(1): 1-6.
Abstract
In the Pandemic of COVID-19 infection caused by SARS-CoV-2, no longer the age and preliminary health status are barriers against this disease-associated morbidity and mortality. In COVID-19 a dysregulated immune response and an exaggerated pro-inflammatory cytokines release are reported. The loss of taste and smell as early alarming
symptoms reflect acute serum zinc deficiency. The pathogenesis can be explained as a redistribution of zinc ion associated with acute immune cellular dysfunction. Zinc deficiency results in multiple immunological changes with a shift towards a predominantly innate immune response, which is not as effective in viral immune clearance as the
adaptive immune response. Notably, micronutrients homeostasis plays a key role in maintaining a healthy immune response especially Vitamin C, Vitamin D, Zinc and Magnesium. Zinc is considered as the gatekeeper of the immune system. Current studies on zinc-ionophores especially, chloroquine and quercetin, reported an effectiveness in the reduction of COVID-19 morbidity and mortality with an early administration. These Zinc-ionophores are able to chelate zinc and concentrate it intra-cellularly. Concerns about chloroquine safety, its pH-dependent efficacy, response polymorphism, and drug-resistance were studied in malaria treatment. Quercetin is a lipid- soluble, naturally occurring flavonoid, available as a dietary supplement with chloroquine-like actions. It is postulated that zinc supplementation combined with zinc-ionophores may offer dual anti-viral and immune-modulatory effects in favor of both the maintenance and the resetting of an effective cell-mediated immune response.
Keywords
Abbreviations List
CD: cluster of differentiation; COVID-19: Corona Virus Disease in 2019; CQ: Chloroquine; DC: Dendritic cell; FEV: Forced expiratory volume; FVC: Forced vital capacity; IL: Interleukin; MHC: Major Histocompatibility Complex; QC: Quercetin; QTc: Corrected QT interval; SARS-CoV: Severe acute respiratory syndromerelated coronavirus; SLM: solid lipid microparticle; Th: T helper; Treg: T regulatory; ZENITH: Zinc Effect in Nutrient/ Nutrient Interactions and Trends on Health and Ageing; Zincage: Zinc and Ageing.
Significance Statement
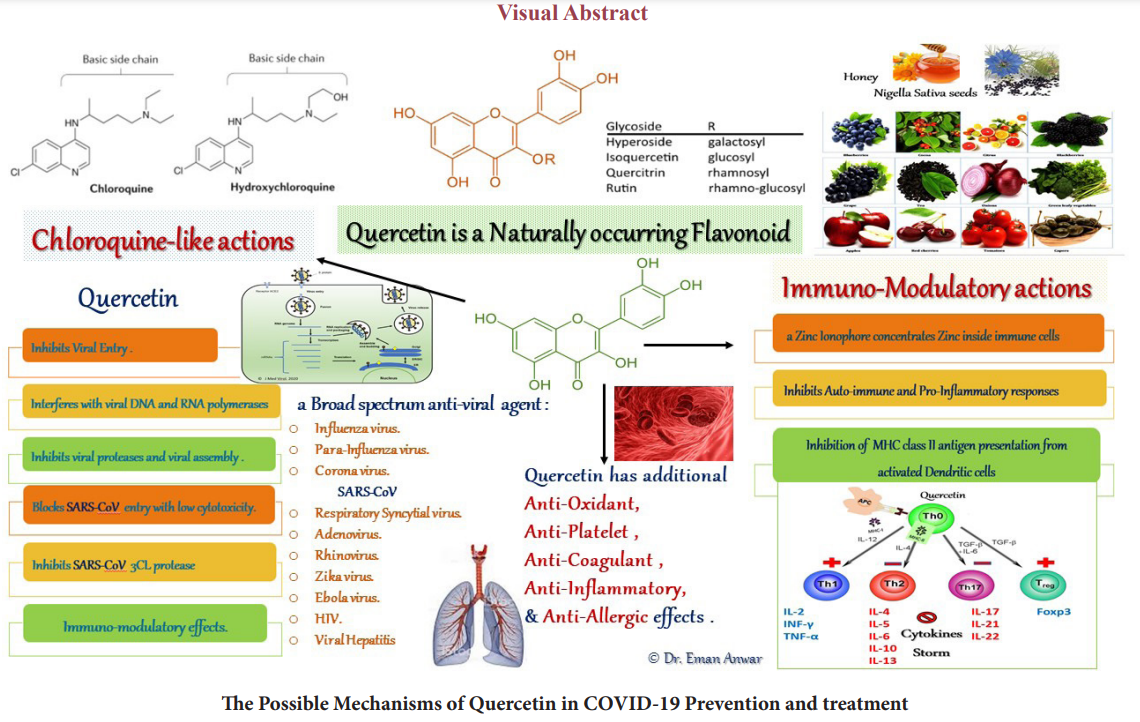
With the world-wide reopening conditions after the lockdown restrictions. Still the virulence of the SARS-CoV-2 isn’t attenuated and the vaccine trials aren’t yet completed. A long-term supplementation with chloroquine as an immune supportive agent isn’t recommended due to safety concerns. Quercetin is a dietary supplement having chloroquine-like actions. Quercetin plus essential immunity vitamins and minerals in their recommended doses may offer an adequate immune support.
Introduction
In spite of the availability of selective antagonists to different steps of SARS-CoV-2 virus replication cycle, there is a background pathogenesis hasn’t yet been adequately targeted. According to the American society for microbiology, a suggested list of agents targeting SARS-CoV-2 intracellular replication steps starting from the endosomal and the non-endosomal viral attachment and entry, the uncoating, proteases processing prior to cytosolic RNA replication then endoplasmic reticulum translation of viral proteins (spike S, membrane M, nucleocapsid N and envelope E) till viral reassembly and release [1].
The normal viral immune response is an integration of innate to adaptive cellular responses. The main effector cells of innate immunity are macrophages, neutrophils, dendritic cells, and natural killer cells, whereas of the adaptive immune system are the T and B lymphocytes. An effective innate immune response is the threshold stimulus to a balanced adaptive one [2,3].
An abnormal immune response is suggested to be in COVID-19 pathogenesis manifesting SARS-CoV-2 virulence. It could be an immune dysregulation explained by some micronutrient’s deficiency [3] and/or a superantigen immune response triggering an abnormally high percent of T cell response to viral antigens, especially the spike S [4]. In SARS-CoV-2 infection, an excessive inflammatory innate response and a dysregulated adaptive response are describing COVID-19 immunopathology [2].
The cross talk among immune cells is orchestrated by micronutrients especially Vitamin C and Vitamin D [3]. The checkpoint or the bridge from innate response to adaptive cellular response is in: the activated dendritic cells to the T cells, antigen presentation balance via major histocompatibility complex (MHC) class subsets [5-7]. Antigen presentation via MHC class- I by DC elicits the T helper (Th)-1 to CD8 response, which is effective in viral clearance, seronegative conversion and a memory T cell for subsequent infection. Whereas, the MHC class-II to Th-2 and B cell activation to plasma cells elicit the humoral immunity with the production of immunoglobulins. The immunoglobulins help in reducing the inter-cellular spread of the virus by its neutralization, and the memory B cells remain for future defense [8-10].
Zinc as a Gatekeeper of Immune System
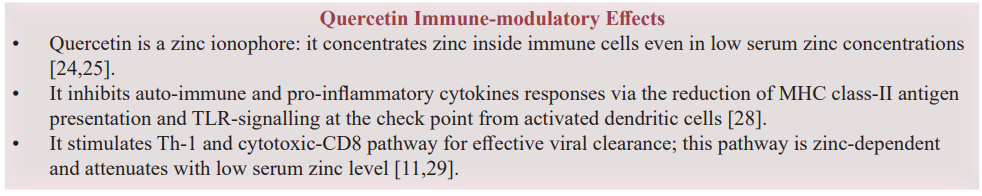
A normal serum zinc level is essential for immune cellular response balance. As described in Ananda S. Prasad 2000, the cytokines release pattern is zinc- dependent. Serum zinc deficiency is associated abnormal Th-1 (CD8+ T cell) to Th-2 (CD4+ T cell) cell populations ratio. A shift occurs from Th-1 -CD8 specific pathway to a Th-2-CD4 and B cell non-specific responses. The Th-2 pathway is less affected in low serum zinc concentrations and with increased pro-inflammatory cytokines release from Th3; namely IL-4,5,6 and 10, whereas the products of Th-1 cells e.g. γ-interferon (IFN-γ) and IL-2 are decreased [3,11]. Zinc deficiency also contributes to an imbalance between autoimmune responses and immune tolerance; an elevation in Th-17 cell numbers associated with autoimmune diseases and a reduction in T-reg cell numbers, which control the immune tolerance [3,9].
On the other hand, zinc overdose suppresses normal T and B cells functions by an overload on T-reg cells [3,9].
In 2012, Henriques et.al reported that the low soil zinc adversely affected the crops and the human serum zinc in developing and developed countries, with a worldwide human zinc deficiency status. European studies ZENITH in 2005 and Zincage in 2006, have addressed the importance of supplementation of zinc in elderly population with beneficial effects on mental and immunity functions in terms of normal lymphocytes proliferation and cytokines release pattern especially in those with IL-6 polymorphism [12]. Zinc supplementation in elderly was shown to restore the T helper subsets balance affected by ageing [13].
An acute zinc redistribution was described as a body defense against the infectious pathogens, to the liver and to the intracellular compartment especially inside immune cells due to the upregulation of zinc import proteins [14,15]. In low dietary zinc intake, a peripheral lymphopenia and a reduction of the lymphocytesÍ? intracellular zinc occur before the decline in serum zinc level below the normal range [11]. The loss of taste and smell sensation is a manifestation of acute zinc deficiency [16], in COVID-19, it may highlight an early acute serum zinc consumption associated with an acute immune cellular dysfunction [2]. The Pattern of MHC classes antigen presentation and their intracellular trafficking in immune cells of both innate and adaptive immune responses are affected in serum zinc deficiency state [3,9,11].
Zinc-ionophores Immuno-modulatory and Anti-viral Effects Chloroquine (CQ), the anti-malarial drug, was included in international protocols treating COVID-19 and an effectiveness was reported with the early administration of either CQ or its safer derivative hydroxychloroquine [17-19]. At cellular level, CQ acts as a zinc ionophore which carries zinc ion through the lipid bilayer to inside the cell independent of the ion channels and serum zinc concentration, its effect increases in a dose dependent manner [20]. Its direct anti-viral effects against both RNA and DNA viruses, attacking many steps in virusesÍ? replication cycles were reported [21]. A direct in-vitro effect on SARS-CoV-2 replication is reported [18].
As a weak base, CQ is concentrated inside the lysosomes, it inhibits the auto-antigenic presentation via MHC class-II and cell autophagy [6]. CQ reduces Toll-like receptors TLR-7 and-9 signalling and the exaggerated pro-inflammatory cytokines production from the innate immune system and the activated DC- Th 2 - B cell pathway [18]. These effects reset the balance of Th3/ Th3 and CD8/CD4 responses ratio.
Potential risks of CQ-treatment include increased risk of retinopathy, prolongation of the QTc interval (especially in patients with pre-existing cardiac disease or if co-prescribed with azithromycin), hypoglycemia, neuropsychiatric effects, drugdrug interactions and idiosyncratic hypersensitivity reactions [5].
Genetic variability in the metabolism of CQ is considerable and influences its safety and effectiveness [19]. CQ-resistance and treatment-failure were also reported due to Plasmodium Malaria genomic mutations and pH-dependent CQ pharmacokinetics [19,22,23].
These concerns about chloroquine treatment are calling for a safer and a mechanistically equivalent alternative, especially if it is widely used in a viral pandemic like COVID-19.
Quercetin (QC) is a naturally occurring flavonoid having a zinc- ionophore activity. It is lipid-soluble and chelates zinc on its carbonyl oxygen (C-3,4 O-) and the deprotonated (C-5 OH) to the intracellular compartment [24,25]. In COVID-19, quercetin was introduced in February 2020 by Eastern Virginia medical school team in combinations with essential immunity vitamins and minerals namely; vitamin C, vitamin D, Zinc, and Magnesium -if needed- in both the prevention and treatment protocols [26].
Michigan university (ISOHIGH) (sph.umich.edu/biostat/covid19_ research), a randomized controlled trial using iso-quercetin in COVID-19 prevention and seronegative conversion on the high- exposure-risk health care personnel. Open-labelled trials are registered in ClinicalTrials.gov using quercetin in COVID-19 prevention and treatment and to date the results haven’t yet been declared.
Broad-spectrum anti-viral effects of QC were studied on many viruses e.g. SARS-CoV-1, Influenza-A, Influenza-A h3N1, Para- influenza, Respiratory Syncytial Virus, Adenovirus, Zika, Ebola, Rhinovirus and other viruses. Quercetin inhibits viral entry of SARS-CoV-1 and the 3C-like protease (3CLpro) and a synergism with vitamin C was suggested. This 3CLpro inhibitory effect of quercetin was dependent on its hydroxyl group [26].
Quercetin has been identified in the supercomputer SUMMIT drug-docking screen (Smith and Smith, 2020), among the top candidate inhibitors of the SARS-CoV-2 spike protein-human ACE2 receptor interface-ligand binding complex [27]. Quercetin had been previously identified as a potent inhibitor of the SARS- CoV-1 infection [27].
Quercetin has additional anti-platelet, anti-coagulant [30,31], anti-oxidant, anti-allergic, and anti-inflammatory effects; non- steroidal and corticosteroid-like actions [29]. An inhibitory effect of Quercetin on angiotensin converting enzyme-2 receptor MAP kinase signaling was described in ischemic heart disease prevention [32]. Studies on Quercetin also reported anti-cancer, nephro-protective and neuro-protective effects [33,34].
Dietary Sources of Quercetin and Historical Aspects
Quercetin is a polyphenolic bio-flavonoid or a plant pigment found in green leafy vegetables and fruits. The sources of dietary QC are onions, garlic, and ginger; fruits such as apple, grapes, cherries and berries; and in tea and dark chocolate. Among the sources of QC are Honey and Nigella sativa seeds [35-37]. However, the concentration of QC as the most abundant flavonoid in honey is variable in honey samples from flowers in different countries [32,35].
Islamic history in Quran and Prophet Mohamed books pointed to” Therein a Cure for Mankind “inside both Honey and Nigella seeds [38,39]. In the Bible and Israelites both Honey and Nigella were considered to be a blessed gift from God [40]. The Ancient Egyptians writings in hieroglyphs on the wall of tombs and museums queen Cleopatra and king Tutankhamun were about Honey, Bees and Nigella as secrets of health and beauty [41,42]. Accordingly, the consumption of Honey and Nigella seed might be higher in middle east countries and in areas using herbal medicine than in other regions [43,44]. The estimated flavonoid dietary intake ranges from 50 to 800 mg/day (QC accounts for 75%), mostly depending on the consumption of fruits and vegetables and the intake of tea [29].
Pharmacology and Safety of Quercetin
Quercetin glycosides are differently absorbed based on the type of sugar attached. Quercetin-dihydrate has the best bioavailability followed by glycosides, aglycone, and finally rutinoside (the major quercetin glycoside in tea). The glucosides are efficiently hydrolyzed in the small intestine by beta-glucosidases to the aglycone form, much of which is then readily absorbed [29,34].
The acidic forms of QC: Quercetin glucuronic acid and its sulfuric acid derivatives were more easily absorbed than quercetin. Importantly, if ascorbate or glutathione levels are insufficient, quercetin is shunted to quercetin-quinone/quinone methide and may exert pro-oxidant effects instead of an anti-oxidant effect [29,45].
Quercetin and its derivatives are initially metabolized by enteric bacteria and enzymes in intestinal mucosal epithelial cells. The concentrations of quercetin metabolites in plasma and liver samples have shown that a higher concentration was found in the liver than in plasma, and the hepatic metabolites were intensively methylated (90%95%). The conjugated QC is bound to plasma proteins especially albumin (99.4%). Quercetin and its metabolites tend to accumulate in the organs involved in their metabolism and excretion, and the mitochondria might be an area of QC concentration within cells. Kidney is a major organ of QC excretion [29]. Randomized controlled trials reported the safety and tolerability in QC doses from 250 to 2000 mg/day. Quercetin use in 1000 mg daily for 12 weeks demonstrated a reduction in upper respiratory tract infection total sick days and in its severity for both middle aged and older subjects [46].
Quercetin was safely tolerated by chronic obstructive pulmonary disease (COPD) patients, in doses up to 2000 mg/day as assessed by lung function; FEV1/FVC, blood profile and COPD assessment test questionnaire [47].
Andres S, et al [48], addressed quercetin possible drug-interactions with cyclosporine, pravastatin and fexofenadine. A recommended caution from the prolonged use of quercetin, in patients with high risk for estrogen-dependent cancer and in patients with renal dysfunction due to the probable intracellular QC accumulation risk.
Inhaled Quercetin, a Potential Hope for Treatment of Viral Pneumonias
An aerosol delivery to the lung of quercetin solid lipid microparticles (SLM) was proposed as a potential active pharmaceutical ingredient for a wide range of respiratory airway diseases especially bronchial asthma and COPD. The effect of non-toxic excipients delivering QC in a suitable particle size to distant small airways had also been highlighted [49,50]. This route and dosage form are suitable in both patients who are intolerant to QC [48] and in viral lung involvement conditions (pneumonia and acute respiratory distress syndrome (ARDS).
The pathogenesis of ARDS was described as an immune dysregulation and exaggerated inflammatory and innate immune responses [51] in which studies on quercetin demonstrated potential targets with its early administration [29].
In advanced ARDS, with the loss of the regenerative ability of alveolar stem cells by the extensive alveolar damage, neither the anti-inflammatory drugs nor the anti-infective agents can reverse the condition and, respiratory failure issues [52], in ARDS advanced cases of acute lung injury, stem cells inhaled therapy reported some promising results.
Conclusion
Viral infections were usually only supported unless complicated. Micronutrients especially vitamins D and C, Zinc and Mg) provide a balanced immune response. COVID-19 late interventions can’t deal with established immune-dysregulation except by selective cytokines- antagonists and corticosteroids. Prevention is as equally important as the treatment. The WHO recommended to reduce the viral load by wearing a face-mask and physical distancing. It is also recommended to support an adequate immune response by either consuming food rich in: quercetin, essential immunity vitamins and minerals, or their supplementation. The deficiency of micronutrients cause a defect in immune system orchestration. The recommended daily doses as approved by the food and drugs administration agency (FDA) are advised to avoid side effects from overdoses or the prolonged supplementation use.
References
- Haiou Li, Yunjiao Zhou, Meng Zhang, et Updated Approaches against SARS-CoV-2. Antimicrobial Agents and Chemotherapy. 2020; 64: 483-520.
- Catanzaro M, Fagiani, F, Racchi M, et al. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Sig Transduct Target 2020; 5: 84.
- Gombart AF, Pierre A, Maggini A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020; 12: 236.
- Cheng MH, Zhang S, Porritt RA, et An insertion unique to SARS-CoV-2 exhibits superantigenic character strengthened by recent mutations. Preprint. bioRxiv. 2020; 21.
- Schrezenmeier E, Dörner Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020; 16: 155-166.
- Ren-Yeong H, Yen-Ling Yu, Wan-Chien C, et Immunosuppressive Effect of Quercetin on Dendritic Cell Activation and Function. Journal of immunology. 2010; 184: 6815-6821.
- Marco Rossi, James W Human Dendritic Cells: Potent Antigen-Presenting Cells at the Crossroads of Innate and Adaptive Immunity. The Journal of Immunology. 2005; 175: 1373-13781.
- Prasad AS. Impact of the discovery of human zinc deficiency on J Am Coll Nutr. 2009; 28: 257-265.
- Kulik L, Maywald M, Kloubert V, et al. Zinc deficiency drives Th37 polarization and promotes loss of Treg cell function. J Nutr 2019; 63: 11-18.
- Elmadfa I, Meyer AL. The Role of the Status of Selected Micronutrients in Shaping the Immune Endocr Metab Immune Disord Drug Targets. 2019; 19: 1100-1115.
- Prasad Effects of zinc deficiency on Th3 and Th3 cytokine shifts. J Infect Dis. 2000; 182: 62-68.
- Mocchegiani E, Romeo J, Malavolta M, et al. Zinc: dietary intake and impact of supplementation on immune function in Age (Dordr). 2013; 35: 839-860.
- Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing 6, 9. Immunity ageing. 2009; 12: 6-9.
- Bin B, Seo J, Kim Function, Structure, and Transport Aspects of ZIP and ZnT Zinc Transporters in Immune Cells. Roles of Zinc and Zinc Mediators in Immunity. Journal Of Immunology Research. 2018; 1-9.
- Alker W, Haase Zinc and Sepsis. Nutrients. 2018; 10: 976.
- Pisano M, Hilas O. Zinc and Taste Disturbances in Older Adults: A Review of the Consult Pharm. 2016; 31: 267-270.
- Robin E, Aronson Jeffrey Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020; 369: 1432.
- Katelyn A Pastick, Elizabeth C Okafor, Fan Wang, et al. Review: Hydroxychloroquine and Chloroquine for Treatment of SARS-CoV-2 (COVID-19). Open Forum Infectious 2020; 15: 4.
- Juurlink Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020; 192: 450-453.
- Xue J, Moyer A, Peng B, et Chloroquine is a zinc ionophore. PLoS One. 2014; 9: 109180.
- Savarino A, Livia Di Trani, Isabella Donatelli, et al. New insights into the antiviral effects of Lancet Infect Dis. 2006; 6: 67-69.
- Golassa L, Erko B, Baliraine FN, et al. Polymorphisms in chloroquine resistance-associated genes in Plasmodium vivax in Malar J. 2015; 14: 164.
- Yayon A, Cabantchik Zvi, Ginsburg Susceptibility of human malaria parasites to chloroquine is pH dependent. Proceedings of the National Academy of Sciences of the United States of America. 1985; 82: 2784-2788.
- Primikyri A, Mazzone G, Lekka C, et Understanding zinc(II) chelation with quercetin and luteolin: a combined NMR and theoretical study. J Phys Chem B. 2015; 119: 83-95.
- Dabbagh-Bazarbachi H, Clergeaud G, Quesada IM, et Zinc Ionophore Activity of Quercetin and Epigallocatechin-gallate: From Hepa 1-6 Cells to a Liposome Model. J Agric Food Chem. 2014; 62: 8085-8093.
- Colunga Biancatelli RML, Berrill M, Catravas JD, et Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front Immunol. 2020; 11: 1451.
- Glinsky GV. Tripartite Combination of Candidate Pandemic Mitigation Agents: Vitamin D, Quercetin, and Estradiol Manifest Properties of Medicinal Agents for Targeted Mitigation of the COVID-19 Pandemic Defined by Genomics- Guided Tracing of SARS-CoV-2 Targets in Human Cells. 2020; 8: 129.
- Huang RY, Yu YL, Cheng WC, et al. Immunosuppressive effect of quercetin on dendritic cell activation and J Immunol. 2010; 184: 6815-6821.
- Li Y, Yao J, Han C, et Quercetin, Inflammation and Immunity. Nutrients. 2016; 8: 167.
- Stainer AR, Sasikumar P, Bye AP, et The Metabolites of the Dietary Flavonoid Quercetin Possess Potent Antithrombotic Activity, and Interact with Aspirin to Enhance Antiplatelet Effects. TH Open. 2019; 3: 244-258.
- Bijak M, Ziewiecki R, Saluk J, et al. Thrombin inhibitory activity of some polyphenolic compounds. Med Chem Res. 2014; 23: 2324-2337.
- Khalil MI, Sulaiman The potential role of honey and its polyphenols in preventing heart diseases: a review. Afr J Tradit Complement Altern Med. 2010; 7: 315-321.
- SK Shebeko, IA Zupanets, OS Popov, et al. Chapter 27-Effects of Quercetin and Its Combinations on Health. Polyphenols: Mechanisms of Action in Human Health and Disease. 2018; 373-394.
- Jaffe R, Mani J. Polyphenolics Evoke Healing Responses: Clinical Evidence and Role of Predictive Polyphenols in Human Health and Disease. 2013; 1: 695-705.
- Yiuchung Cheung, Maninder Meenu, Xiaoming Yu, et Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. International Journal of Food Properties. 2019; 22: 290-308.
- Elkhayat ES, Alorainy MS, El-Ashmawy IM, et al. Potential Antidepressant Constituents of Nigella sativa Pharmacogn Mag. 2016; 12: 27-31.
- Merfort V Wray, HH Barakat, SAM Hussein, et al. Flavonol triglycosides from seeds of Nigella sativa. Phytochemistry. 1997; 46: 359-363.
- Dr Tauseef Ahmad Khan, The University of Toronto, Honey: A cure for mankind? 2019.
- Musharraf HM, Arman Prophetic medicine is the cheapest, safest and the best remedy in the prevention and treatment of hypertension (high blood pressure) a mini review. Int J Mol Biol Open Access. 2018; 3: 245-250.
- Patrick Honey. A healing gift from the Creator. Creation. 2015; 37: 1417.
- Padhye S, Banerjee S, Ahmad A, et From here to eternity - the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008; 6: 495-510.
- Iman Kandil. The Story Behind the Hype: Black Seed Oil. Nigella 2017.
- Muhammad Zakariyyah Aumeeruddy, Hudaa Neetoo, Mohamad Fawzi Mahomoodally. Journal of Complementary Medicine Research, 2018; 8: 1527.
- Sarina Mohamad, Hayati Mohd Yusof, Nor Jana Salim, et The Effects of Nigella sativa Seeds and Honey Mixture on Lipid Profile: Gender Comparison. Journal of Basic and Applied Scientific Research. 2015; 5: 38-42.
- Boots AW, Kubben N, Haenen GR, et al. Oxidized quercetin reacts with thiols rather than with ascorbate: implication for quercetin supplementation. Biochem Biophys Res Commun. 2003; 308: 560-565.
- Heinz SA, Henson DA, Austin MD, et Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol Res. 2010; 62: 237-242.
- Han MK, Barreto TA, Martinez FJ, et Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2020; 7: 392.
- Andres S, Pevny S, Ziegenhagen R, et al. Safety Aspects of the Use of Quercetin as a Dietary Mol Nutr Food Res. 2018; 62: 10.
- Piyush Mehta, C Bothiraja, Kakasaheb Mahadik, et Phytoconstituent based dry powder inhalers as biomedicine for the management of pulmonary diseases. Biomedicine & Pharmacotherapy. 2018; 108: 828-837.
- Scalia S, Haghi M, Losi V, et Quercetin solid lipid microparticles: a flavonoid for inhalation lung delivery. Eur J Pharm Sci. 2013; 49: 278-285.
- Lin S, Wu H, Wang C, et al. Regulatory T Cells and Acute Lung Injury: Cytokines, Uncontrolled Inflammation, and Therapeutic Front Immunol. 2018; 9: 1545.
- Horie S, Gonzalez HE, Laffey JG, et al. Cell therapy in acute respiratory distress syndrome. J Thorac Dis. 2018; 10: 5607-