Metastatic Colorectal Cancer: Redox Metabolism and Malignancy
Author'(s): Burlaka A.P1*, Yevtushenko O.I3, Burlaka A.A2, Yatsyna O.I2, Lukin S.N.1 and Ganusevich I.I.1
1R.E. Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology NAS of Ukraine, Kyiv, Ukraine.
2Ukrainian National Cancer Institute, Kyiv, Ukraine.
3Shupyk National Medical Academy of Postgraduate Education,Kyiv, Ukraine.
*Correspondence:
Burlaka A.P, R.E. Kavetsky Institute of Experimental Pathology,Oncology and Radiobiology NAS of Ukraine, Kyiv, Ukraine,Tel: +38067-683-45-16.
Received: 20 July 2021; Accepted: 29 August 2021
Citation: Burlaka AP, Yevtushenko OI, Burlaka AA, et al. Metastatic Colorectal Cancer: Redox Metabolism and Malignancy. CancerSci Res. 2021; 4(2): 1-7.
Abstract
Investigating the tumor microenvironment has a direct impact on the understanding of the mechanisms which regulate cancer progression and treatment. Tumor cells undergoing profound changes in their own intrinsic metabolism affect the microenvironment. Colorectal cancer (CRC) is the third most common cancer with the predominant metastases (Mts) into the liver. Mts in liver tissues (LT), adjacent to Mts (AMT) and remote tissues (RMT, taken at the distance of 5 cm from Mts) from 25 patients with metachronous liver Mts after the liver resection as well as blood and urine were studied by electron paramagnetic resonance (EPR) including the spin-trapping EPR for the detection and quantification of the activity of N2 iron-sulfur proteins, levels of NO-N2 complexes, labile iron pool (LIP), lactoferrin (Lf), superoxide and NO radicals. Activity of metalloproteinase MMP-2 and MMP-9 were determined. In adjacent and remote LT low activity of N2 in mitochondrial electron transport chain (EPR signal with g=1.94), loss of functions of detoxification system (cytochrome P450, g=2.25), appearance and growth of NO-N2 complexes (g=2.007) are obtained. Intensive EPR signals from LIP (g=2.2-2.4) and Lf (g=4.3) are registered. Superoxide generation rates were of up to 6 times higher than in the reference LT tissues and blood (p<0.001). NO levels are of 1.7 times higher for the AMT compared to RMT (p<0.05) while being 15 times higher for blood comparing to the reference species (p<0.001). Activity of MMP-2 and MMP-9 was registered both in AMT and RMT and is in 1.7 times higher in ALT (p<0.05). The obtained results can be used to estimate the functional state of organs and tissues with distant metastases, the risk of recurrence, to correct the antitumor therapeutic procedures.
Keywords
Introduction
Colorectal cancer (CRC) is the third most common cancer in both sex with more than a million of new cases per year and more than 500,000 deaths registered worldwide with few treatment options especially for advanced and metastatic patients [1-3]. The liver is the most common site of metastasis in people with CRC; over 50% of patients with CRC metastases (mCRC) have liver-only metastases [4]. Metastatic colorectal cancers remain incurable [5].
The development of CRC is dependent on several mechanisms. Genes that affect the control of cell growth are frequently mutated in colon cancer. In CRC, free radicals produced by the colonic bacteria Enterococcus faecalis may directly cause mutations in colonic DNA resulting in colon cancer. Free radicals convert dietary procarcinogens to carcinogens that may contribute to CRC. DNA damage caused by reactive oxygen species (ROS) is a major contributor to CRC development in ulcerative colitis patients. Thus, oxidative stress induced cellular damage may provide a mechanistic basis for colon cancer by causing genetic instability, specific gene alterations, and aberrant methylation [6,7].
It is known that ROS generated by cancer cells and cells in cancer microenvironments can guide and maintain the growth and differentiation of local and distant cancers and their interactions with host microenvironments [4-10], but their roles in pathophysiology and disease pathogenesis have not been well studied. Generally, it is assumed that low and moderate amounts of ROS have beneficial effects on several physiological processes including killing of invading pathogens and tissue repair processes. ROS act as essential signaling molecules. Cancer treatment by chemotherapeutic agents and radiotherapies depend largely on ROS generation to destroy malignant cells by inducing apoptosis. However, disproportionate generation of ROS poses a serious problem to bodily homeostasis and causes oxidative tissue damage [6-12].
Cellular redox state is associated with the synthesis and degradation of matrix proteins, with consequent effects on cell survival, invasion, and metastasis. Matrix metalloproteinases (MMPs) are capable of decomposing extracellular matrix proteins, enhancing invasion of cancer cells. Generally, it is assumed that the synthesis and/or activation of MMP is increased by oxidative stress such as that created by activated neutrophils and ROS, for example [13].
Recent data suggest more important and significant roles for neutrophils and platelets in tumor biology. Neutrophil-to- lymphocyte, platelet-to-lymphocyte ratios have been proposed as independent markers of poor prognosis in patients with cancer, including CRC [14,15]. Human and animal studies have led to the suggestion that there are two neutrophil phenotypes, so called antitumour N1 neutrophils and pro-tumorigenic N2 neutrophils. The N1 neutrophils show a direct antitumour effect induced by ROS production as well as antibody-dependent cellular cytotoxicity. Meanwhile, N2 neutrophils are thought to facilitate cancer development via reconstruction (reprogamming) of the extracellular matrix, acceleration of angiogenesis and lymphangiogenesis and immune modulation through pro- tumorigenic cytokine production [16-19].
In this work, we study the features of redox state in liver metastases (Mts), their microenvironment, in the blood and urine of patients with metastatic CRC (mCRC) with aim to shed light on mechanisms of formation of a metastatic microenvironment.
Materials and Methods
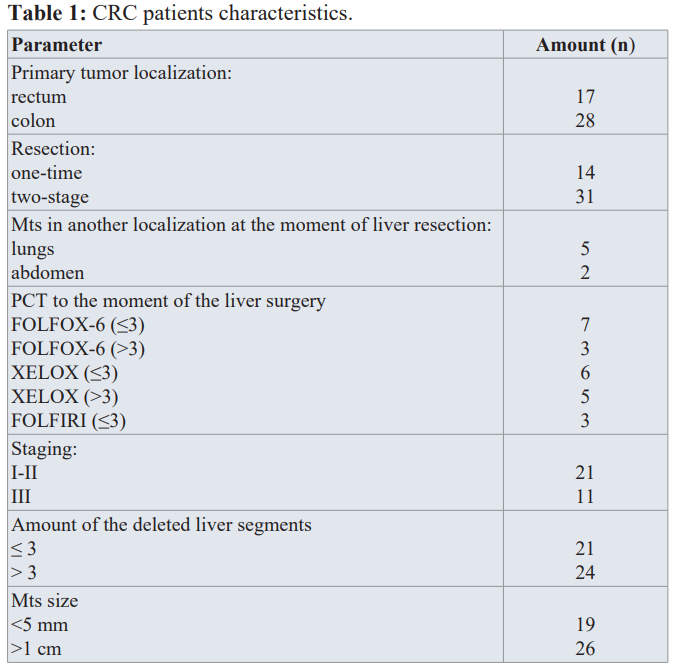
Liver tissues (LT) of 45 patients (24 male, 21 female), average age of 59 ± 2.3 y.o with metachronous colorectal liver metastases (pT1-4N0-2M0-1 for colon cancer and pT1-3N0-2M0-1 for the rectal cancer in pathologic staging) who were treated at the National Cancer Institute (Ukraine) in the period from 2016 to 2020 were investigated. All participants expressed their prior written consent to take part in the research and to use the tissues obtained by surgery for the research purposes. The study was approved and guided by the Bioethical Committee at the R.E. Kavetsky Institute in accordance with Helsinki declaration (1964 and later amendments). The detailed patient characteristics and samples studied are given in Table 1.
The patients underwent polychemotherapy (PCT) in compliance with international, domestic standards and to clinic protocols during the mentioned period. LT (biopsy material) taken from 11 patients with benign liver neoplasm, who did not underwent PCT, did not have hepatitis or any other liver diseases in anamnesis, were considered as control (reference) species. Blood and urine of CRC patients (n=12) with M0 (5 patients with stage II, 7 with stage III, N1-2), and control samples of almost healthy donors (n=11) were analyzed.
The tissue species obtained during the liver resections were frozen and kept in special molds in liquid nitrogen (T = 77 K). Electron paramagnetic resonance (EPR) measurements were conducted at room (RT) and liquid nitrogen temperatures on RE- 1307 (USSR) and ESP-300 (Bruker, Germany) X-band (9 GHz) spectrometers. For the RT studies the samples were then unfrozen. The spectrometers handling, data collection and analysis were carried out with the homemade personal computer interfaces and self-written software.
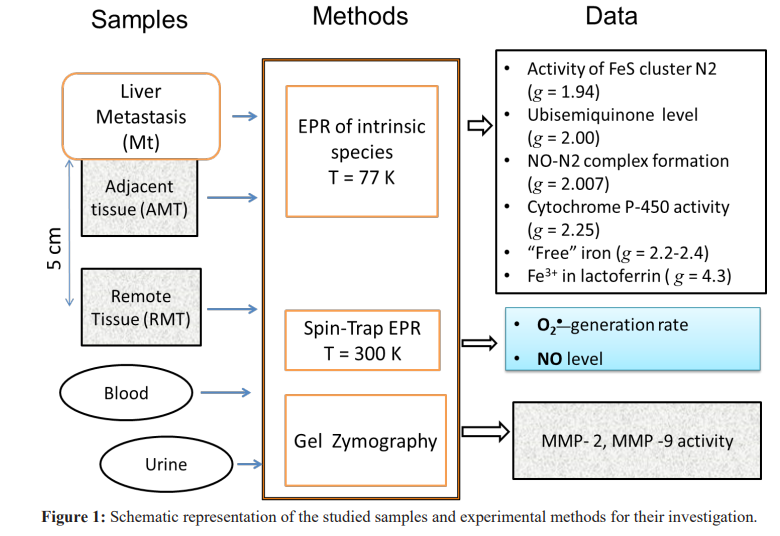
Spin traps 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine hydrochloride (TEMPONE-H, Sigma-Aldrich, USA) and Fe/ diethyldithiocarbamate (Fe/DETC) were exploited to extract the rates of superoxide O • generation and nitric oxide NO concentrations in the investigated tissues, correspondingly, at RT by EPR. The technical details of EPR measurements of the intrinsic and trapped paramagnetic centres along with the sample preparations are given in our previous papers [20-22]. Figure 1 schematically summarizes materials, methods and experimental parameters extracted from our research.
Concentrations of MMP-2 and MMP-9 in samples both in active and latent forms were determined by gelatine zymography - the polyacrylamide gel electrophoresis-based method with using sodium dodecyl sulfate (SDS). The details of the corresponding analysis are given in [23]. Activity of 1 µg of ferment in 1 ml of the control sample of the blood serum and 1 µg of ferment on 1 g of creatinine in urine were set as a corresponding arbitrary unit (a.u.).
Results and Discussion
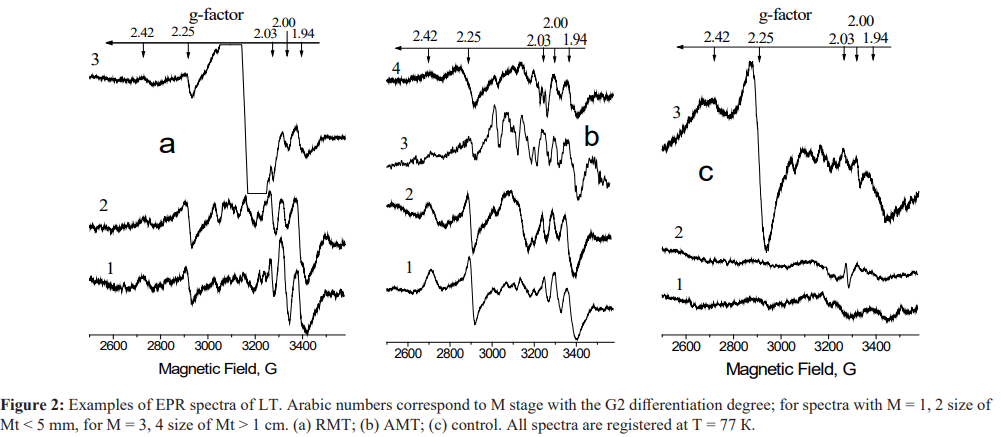
The results of the EPR measurements of the intrinsic paramagnetic centers in the investigated species are presented in Figure 2 and Tables 2and3. It is known (see [8,20,23] and references therein) that EPR is sensitive to the changes in the mitochondrial electron transport chain (ETC). Signal with g = 1.94 is related to the activity of the iron-sulfur cluster N2 of NADH: ubiquinone oxidoreductase, also called respiratory complex I. Signal with g 2.00 is ascribed to the “free” radical centers practically completely localized in mitochondria - semiquinones of flavoproteins found in the inner membrane of mitochondria and coenzyme Q semiquinones (ubisemiquinones). Signal with g = 2.03 characterizes a generation of NO and N types FeS-protein complexes Signals with g = 2.25 and g = 2.42 are connected with the catalytic cycle of the cytochrome P450 which serves as a natural detoxifying agent of the body. One of the EPR labels of the pathological changes is an appearance of “triplet” signal with g ≈ 2.007. Its origin is caused by the nitrogen hyperfine interaction with the iron ions in nitrosyl-iron protein complexes of hemoproteins and cytochromes. The EPR marker of the malignant tumor growth was revealed in the tumor tissues
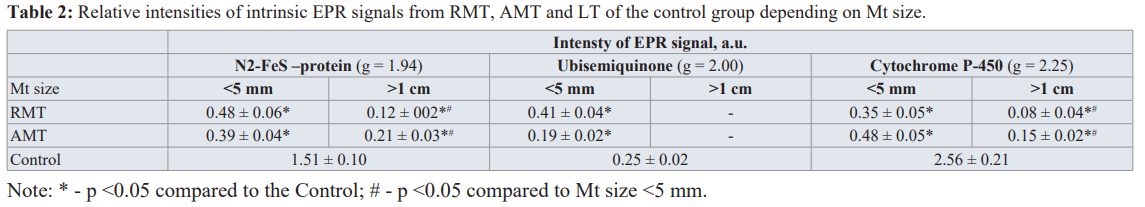
Figure 2(a) presents the results for RMT at different M stages (1-3) with the differentiation degree G2. The parameters of these remote from Mts tissues from the first glance should be close to the normal (control) species. However, even in those tissues the changes are obvious (see Table 2). They include a decrease in the activity of FeS proteins N2 in ETC of hepatocytes (g = 1.94) which indicates noticeable decrease in the signals from N2 FeS-proteins and P450 cytochrome independently on the distance from Mt and its size. In presence of Mts with the diameter >1 cm, signals from the N2 FeS- protein and P450 cytochrome are reliably less intensive than in the presence of Mts with the diameter < 5 mm. Activity of cytochrome P450 in RMT correlates negatively with the size of Mts (r = -0.64; Ñ? <0.05). The level of ubisemiquinone in the presence of Mts with the diameter < 5 mm increases in RMT and decreases in AMT. We suppose that all these changes in the redox state of LT under the influence of metastases lead to the unregulated increase in the O •
a defect in mitochondrial oxidative phosphorylation, in terms of a reduction in the activity of NADP·H-CoQ reductase complex. An increase of EPR signal with g ≈ 2.00 (ubisemiquinones) is to obtain for spectrum 1 while for spectrum 2 in Figure 2(a) it is practically the same as in the control group (Table 2). It can be explained by different duration of exposure of Mt and its microenvironment on LT. Disturbances in the functioning of the cytochrome P450 redox cycle are manifested as a decrease in the EPR signal intensity (g= 2.25) that becomes extremely evident for the metastasis size 1 cm (spectrum 3).EPR spectra for AMT at different M stages (1-4) with the differentiation degree G2 are presented in Figure 2(b). For Mt size< 5 mm a decrease in the activity of N2 FeS proteins, a decrease in the level of ubisemiquinone, cytochrome P450, and a compensatory growth of the activity of ubiquinone-cytochrome c-reductase and Rieske FeS-center (g = 2.10, spectrum 2) are registered. For Mt size 1 cm (spectra 3, 4) an increase in the levels of the cell hypoxia marker - NO-FeS proteins in Complex I of the mitochondrial ETC (g = 03), a decrease in the activity of the N2 FeS-protein and the level of ubisemiquinone is accompanied by formation of “triplet” signal with g ≈ 2.007 (spectrum 4), low-molecular complexes of Mn2+ (six lines in the magnetic field ranging from 3100-3400 G, spectrum 3). A long exposure of Mts onto the surrounding lever tissues (spectra 3,4) causes further intensification of the processes of reprogramming of the mitochondrial oxygen metabolism, to the complete loss of the active form of the redox cycle for P450 cytochrome.
Examples of EPR spectra for Mt tissues themselves are given in Figure 2(c). Spectra 1 and 2 are typical for malignant cells [8,23] with the low oxidative phosphorylation level and absence of signal(s) from cytochrome P450. Spectrum 3 of MN also has a spectral signs of formation of malignant cells that may be identified by the anomalously high activity of P450 cytochrome (g = 2.25).
As it follows from the analyses of the discussed above results which are gathered in Table 2, the presence of Mts in liver leads to the generation rate.
Iron is an essential element that promotes the proliferation of cells and their growth. Ions of "free" iron (labile iron pool, LIP) take part in redox reactions in cells, forming their redox state. The nature of the intensive broad EPR signal at g ≈ 2.2-2.4 that appears in the tumor affected tissues (cf. spectrum 3 in Figure 2a) is connected with the ferritin molecules or non-ferritin (anti) ferromagneticaly ordered iron-containing (nano)particles [24-27]. Lactoferrin (Lf) is a multifunctional iron-containing glycoprotein within the iron-binding transferring (Tf) family, which is produced by glandular cells and neutrophils in infected tissue and blood during the inflammatory process. Lf (with the iron on its surface that can interact with the molecular and cellular components of the body and pathogens) is one of the main components of the innate immune system [28-30]. Lf regulates bioavailability of iron in realization of cells’ metabolic functions, specifically proliferation. So, “free” iron and Lf (with the EPR signal corresponding to the complexes of Fe3+ ions at g ≈ 4.3) can be attributed to the important factors of tumor growth, their microenvironment and metastasis [31-33]. Results of the EPR measurements for “free” iron (g = 2.2- 2.4) and Lf (g = 4.3) EPR signals in Mt, AMT and RMT tissues are presented in Table 3. In AMT both iron signals are more intensive than in RMT.
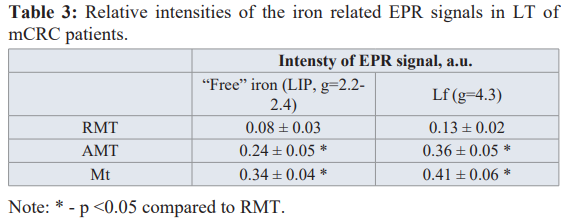
The appearance of LIP can be due to the decompartmentalization of iron ions because of the activation of oxidative damages of lipids, proteins, membranes and destruction of iron-containing proteins, a transformation of Tf to apo-Tf and the release of the deposited iron from ferritin. “Free” iron is an effective promoter of radical reactions and it catalyzes formation of superoxide and hydroxyl radicals supporting oxidative phenotype [29-31].
From Table 3 it follows that contact of Mt with LT causes a significant increase of Lf signal in AMT compared to RMT. It is worth to note that in control LT no Lf or LIP signals were detected. Therefore, an increase of Lf and/or “free” iron signals in LT can be used as additional markers for the distant metastatic sites.
Data for the superoxide generation rate and NO level in Mts and LT acquired by using spin-trap EPR at RT are listed in Table 4. It follows that O generation rate in AMT and RMT are much higher than in the control group and Mt tissue generate superoxide radicals slower than AMT and RMT. Therefore, mitochondria of LT cells, Mt cells and their environment function in circumstances of a damaging superoxide influence [34].
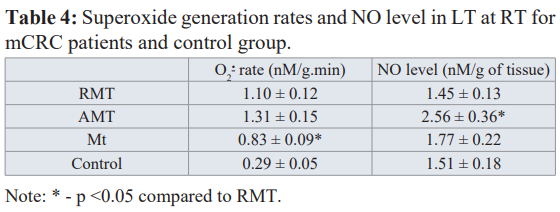
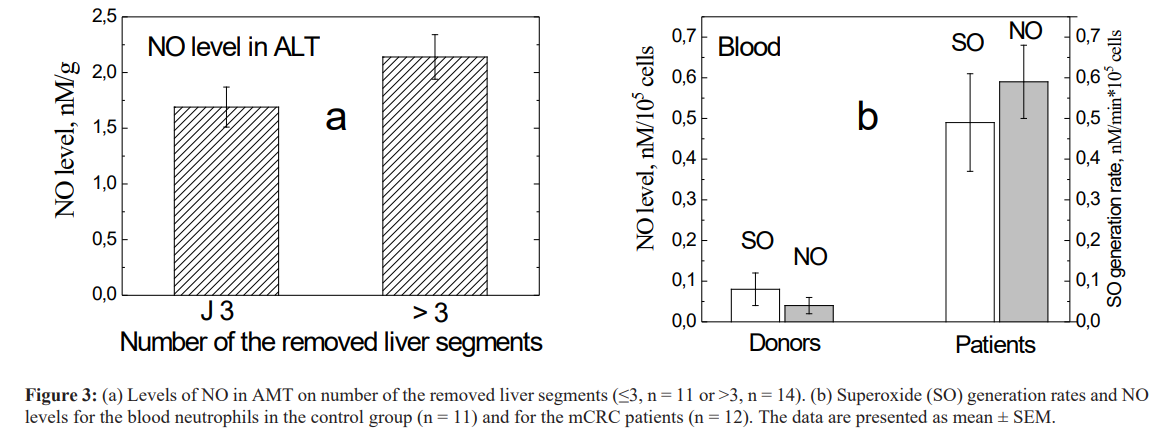
The levels of NO in Mt and RMT do not reliably differ from the control value while its growth in AMT is obtained (Table 4). We found that the level of NO in AMT directly (r = 0.68, p = 0.05) correlates with the number of the removed liver segments (i.e. with the amount of the formed Mt, see Fig. 3a). It is established that NO plays an important role in angiogenesis/ lymphogenesis [35, 36].
NO generated by iNOS in neutrophils is a mediator for the VEGF- induced lymphogenesis. This indicates that NO is involved at the earlier stages of metastasis and high NO levels can be associated with the progression of the malignant processes. All three isoforms of NO synthase (nNOS, iNOS and eNOS) are expressed in the presence of gastrointestinal tract tumor. High levels of iNOS and eNOS expressions were detected for mCRC patients. Tumor angiogenesis is important for progression of a tumor process, it leads to metastasis and provide the proliferation due to development of blood vessels through VEGF activation [36].
We also examined O and NO generating activity of neutrophils of peripheral blood of mCRC patients. The results are presented in Fig. 3b and show that the neutrophils of these patients generate superoxide radicals 6 times faster and NO level is 15 times higher (p<0.001) than in the blood samples of control group. These mean that neutrophils in blood are activated in the presence of liver CRC metastases highlighting the significant role of neutrophils in the progression of a tumor mediated through the effect of angiogenesis and immunosuppression.
The results for the MMP activity measurements are gathered in Table 5. It is important to note that MMP-2 and MMP-9 activity was detected in all samples including RMT. Activities of MMP-2 and MMP-9 in AMT is in 1.7 times and in 1.8 times higher than those in RMT, correspondingly (p<0.05). Activity of MMP-9 in Mt is almost twice higher than its activity in RMT. Therefore, high rates of extracellular matrix (ECM) destruction are observed in Mt and AMT but even in RMT the high levels of proteolytic activity is observed. This indicates significant changes in the ECM structure organization of tissues in organs with metastases and demonstrates the pro-tumor phenotype of the microenvironment at the secondary site when the primary tumor creates favorable conditions for its spread through the corresponding signaling pathways [37-39].
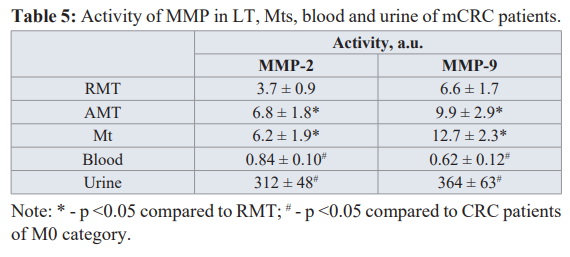
No sensible MMP activity was registered in the blood serum and urine in the control group. There is almost no difference between MMP-9 activity in blood serum and urine of mCRC patients and those in patients with M0 (p > 0.1) while MMP-2 activity in blood and urine of mCRC patients is 2.0 and 1.7 times lower as compared to patients with СRC category M0 (1.71 ± 0.20 and 530 ± 65, respectively). Previously we have shown that MMP-2 activity in tumor tissue of stomach cancer patients with the removed metastases is less than that activity for patients without metastasis [23]. It can be concluded that MMP-2 of a primary tumor plays an important role at the earliest stages of metastasis, i.e. takes place in dissemination of tumor cells.
Conclusion
Defects in ETC of liver tissues adjacent to methastasis and at the distance of 5 cm from that are detected as a result of the mitochondrial dysfunctions and changes in the electron transport accompanied by superoxide generation. Activation of the sources of redox molecules in metastatic and adjacent to Mt tissues as compared to the remote from Mts tissues is revealed. The creation of the metastatic microenvironment in the liver is provided by superoxide and NO activation of MMP and remodeling of the intercellular matrix as well as by the increase of the lactoferrin level and the accumulation of “free” iron, which serve as a signal for the activation of proliferative processes in cells. The obtained redox indicators can be used to estimate the functional state of organs and tissues with distant metastasis, the risks of recurrence, to improve the procedures of the modern antitumor therapy.
References
- Jacques Ferlay, Colombet M, Soerjomataram I, et al. Bray Estimating the global cancer incidence and mortality in 2018 GLOBOCAN sources and methods. Int J Cancer. 2019; 144: 1941-1953.
- Fedorenko Z, Goulak LO, Gorokh YL, et Cancer in Ukraine incidence mortality activities of oncological service. Bulletin of national cancer registry of Ukraine. 2018; 16.
- Kaprin AD, Starinsky VV, Petrova Malignant neoplasms in Russia in 2015. Hertzen Research Institute of Oncology. Moscow. 2017; 250.
- Crotti S, Piccoli M, Rizzolio F, et al. Extracellular matrix and colorectal cancer how surrounding microenvironment affects cancer cell behavior. J Cell 2017; 232: 967-975.
- Peddareddigari VG, Wang D, DuBois The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010; 3: 149-166.
- Bhattacharyya A, Chattopadhyay R, Mitra S, et al. Oxidative stress an essential factor in the pathogenesis of gastrointestinal mucosal Physiol Rev. 2014; 94: 329-354.
- Rosa Vona, Lucia Pallotta, Martina Cappellett, et al. The Impact of Oxidative Stress in Human Pathology Focus on Gastrointestinal Antioxidants. 2021; 10: 201.
- Burlaka AP, Sidorik EP. Redox-dependent signal molecules in mechanism of tumor Naukova dumka Kyiv. 2014.
- Li S, Tan HY, Wang N, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015; 16: 26087-
- Burlaka AP, Ganusevich II, Lukin SN, et al. Superoxide-and NO-dependent mechanisms of the reprogramming of bone marrow cells by tumor cells. Appl Magn Reson. 2014; 45: 1261-1273.
- Sounni NE, Noel A. Targeting the tumor microenvironment for cancer therapy. Clin Chem. 2013; 59: 85-93.
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver J Gastroenterol Hepatol. 2011; 26: 173-179.
- Magnus Ågren. Ulrich auf dem Keller Matrix Metalloproteinases How Much Can They Do. Int J Mol Sci. 2020; 21: 2678.
- Pedrazzani C, Mantovani G, Fernandes E, et al. Assessment of neutrophil-to-lymphocyte ratio platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci 2017; 7: 1494.
- Ishizuka M, Nagata H, Takagi K, et Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013; 109: 401-407.
- Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor Cancer Microenviron. 2015; 8: 125-158.
- Liang W, Ferrara The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016; 4: 83-91.
- Burlaka AP, Ganusevich II, Golotyuk VV, et al. Correlation between superoxide and NO-generating activity of neutrophils of patients with colorectal cancer and clinical characteristics and impact on the long-term results of combined treatment. 2016; 18: 294-299.
- Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal Nat Rev Gastroenterol Hepatol. 2018.
- Burlaka AP, Ganusevich II, Gafurov MR, et Electron paramagnetic resonance study of tumor affected bone marrow. Cancer Microenviron. 2013; 6: 273-276.
- Burlaka A, Selyuk M, Gafurov M, et Changes in mitochondrial functioning with electromagnetic radiation of ultra-high frequency as revealed by electron paramagnetic resonance methods. Int J Rad Biol. 2014; 90: 357-362.
- Burlaka AP, Gafurov MR, Iskhakova KB, et Electron paramagnetic resonance in the experimental oncology implementation examples of the conventional approaches. Bio Nano Science. 2016; 6: 431-436.
- Burlaka AP, Ganusevich II, Gafurov MR, et Stomach cancer interconnection between the redox state activity of MMP-2 MMP-9 and stage of tumor growth. Cancer Microenviron. 2016; 9: 27-32.
- Yurtaeva SV, Efimov VN, Silkin NI, et al. Magnetic Resonance of Ferritin Crystalline Particles in Tumor Tissue. Appl Magn 2012; 42: 299-311.
- Proteasa EA, Schianchi G, Giori EPR detection of possible superparamagnetic polyiron nanoparticles and free radicals in the blood serum of patients with homozygous β-thalassemia. Appl Magn Reson. 2014; 45: 537-571.
- Yurtaeva SV, Efimov VN, Yafarova GG, et EPR detection of iron storage in rat tissues after simulated microgravity model. Appl Magn Reson. 2016; 47: 555-565.
- Gabbasov B, Gafurov M, Starshova A, et al. Conventional pulsed and high-field electron paramagnetic resonance for studying metal impurities in calcium phosphates of biogenic and synthetic J Magn Magn Mater. 2018.
- Burlaka AP, Golotyuk VV, Vovk AV, et Redox state markers of tumors in patients with colorectal cancer. Medical and Clinical Chemistry. 2017; 18: 39-44.
- Torti SV, Torti Iron and cancer more ore to be mined. Nat Rev Cancer. 2013; 13: 342-355.
- Muñoz M, García-Erce JA, Remacha ÁF. Disorders of iron Part II iron deficiency and iron overload. J Clin Pathol. 2011; 64: 287-296.
- Xue X, Shah Intestinal iron homeostasis and colon tumorigenesis. Nutrients. 2013; 5: 2333-2351.
- Pusatcioglu CK, Nemeth E, Fantuzzi G, et al. Systemic and tumor level iron regulation in men with colorectal cancer a case control study. Nutr 2014; 11: 21.
- Tsuda H, Kozu T, Iinuma G, et Cancer prevention by bovine lactoferrin from animal studies to human trial. Biometals. 2010; 23: 399-409.
- Vidal-Vanaclocha F. The prometastatic microenvironment of the Cancer Microenviron. 2008; 1: 113-129.
- Sainz RM, Lombo F, Mayo JC. Radical decisions in cancer: redox control of cell growth and Cancers. 2012; 4: 442- 473.
- Choudhari SK, Chaudhary M, Bagde S, et Nitric oxide and cancer a review. World J Surg Oncol. 2013; 11: 118-127.
- Burlaka, Virko SV, Burlaka AA, et al. Cytochrome P450 content in primary tumors and liver metastases of patients with metastatic colorectal Exp Oncol. 2020; 42: 1-3.
- Colangelo T, Polcaro G, Muccillo L, et al. Friend or foe the tumour microenvironment dilemma in colorectal BBA-Rev Cancer. 1867; 1-18.
- Burlaka AP, Gafurov MR, Burlaka AA, et al. Characteristics of Redox-Metabolism in Patients with Metastatic Colorectal Cancer Clinical Cancer Sci Res. 2020; 3: 1-7.