Novel Delivery System, Alternative to the Vaginal Applicator to Treat Bacterial Vaginosis and Candida
Author'(s): Shihata Alfred A., M.D1*, Brody Steven, M.D, PhD2
1Department of Medicine and Science, Scripps Research San Diego, USA.
2School of Medicine, University of California, San Diego, USA.
*Correspondence:
Shihata. Alfred A, Department of Medicine and Science, Scripps Research San Diego, 14058 Mira Montana Drive Del Mar California USA, Tel: 858 922 7673.
Received: 30 November 2019 Accepted: 27 December 2019
Citation: Shihata. Alfred A, Brody Steven. Novel Delivery System, Alternative to the Vaginal Applicator to Treat Bacterial Vaginosis and Candida. Gynecol Reprod Health. 2019; 3(6): 1-3.
Abstract
Introduction: Vaginal applicators are used for centuries, to deliver creams and gels, into the vagina. The physiologic rhythmic contraction of the vagina is responsible for quick expulsion of menstrual fluid and cervical secretions as well as therapeutic vaginal creams which are rendered less effective.
Objectives: (A) To Provide women with an alternative to vaginal applicators, to maintain therapeutic vaginal preparations in contact with cervix for longer time.
(B) Explore the feasibility for treatment of Bacterial Vaginosis, Candida infections; as well as some STIs by using a new cervical barrier, called FemCap.as a delivery system for antibacterial and antifungal preparations.
Methodology: To prove this concept we recruited 20 women to insert a stained gel with, Gentian violet, using the vaginal applicator and another 20 women to use the FemCap to insert same gel into their vaginas. We then compared the retention and distribution of the stained gel over the cervix. Participants were provided with pads to monitor the expulsion of the stained gel. We also photographed the cervices at 12 and 24 hours.
Results: None of the participants reported any side effects using the stained gel with either device. Women reported leakage while using the vaginal applicator and did not with FemCap. Women who used the applicator had no visible stained gel over the cervix after 12 hours of insertion. Cervical photographs have shown that the FemCap participants retained the stained gel on the cervix more than 24 hours after insertion.
Conclusion: This pilot study has proved the concept, that FemCap is more efficient than the vaginal applicator in delivering vaginal preparations to cervix. The FemCap can shield the cervix from sperm penetration and potentially from STI organism’s invasion. Further studies should be conducted to explore the possibility to treat Bacterial Vaginosis, Candida topically, and some STI’s, such as gonorrhea and chlamydia.
Keywords
Introduction
Some women in the western world self-diagnose and treat their vaginal discharge with antifungal preparations. Most of these women misdiagnose any vaginal discharge as a yeast infection. They waste hundreds of millions of dollars, pollute the environment, and delay their proper diagnosis. A fair percentage of these women have a normal discharge, but some may have Bacterial Vaginosis or sexually transmitted infections, yet all are erroneously treated by the same over the counter antifungal preparations.
The advantages of topical treatment for localized vaginal and cervical infections such as Gonorrhea and Chlamydia are as follows [1]:
- We can use a smaller dose that would be more effective than a larger systemic dose, which comes with side effects.
- Topical vaginal application avoids oral digestive enzymes that may degrade the medication and bypass the liver’s metabolism.
- Under the control of the user [2].
- Does not interfere with intercourse.
Vaginal applicators have been used for centuries to deliver creams and gels into the vagina. The physiologic, rhythmic contraction of the vagina expels the menstrual fluid and cervical secretions quickly, as well as any therapeutic vaginal creams. Vaginitis and cervicitis [3] are usually treated by creams delivered with the vaginal applicator or systemic drugs that come with side effects. The few infections that are treated topically by gels or creams that are inserted by vaginal applicator. Unfortunately, the vagina expels these gels or creams shortly after insertion, rendering them less effective.
Materials and Methods
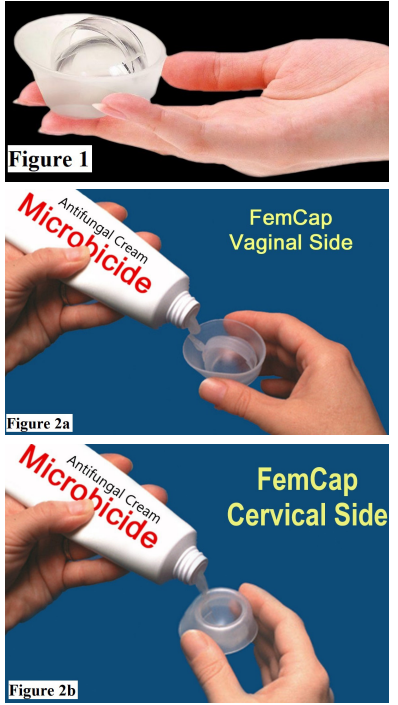
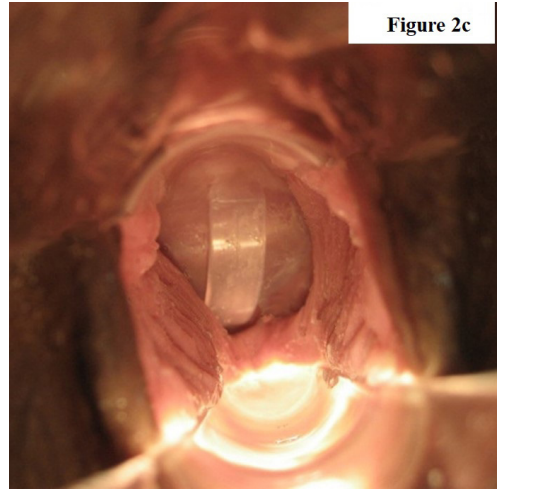
MaterialsA.The FemCap as a Novel delivery system, (Figures 1 & 2a-2b & 2c).
The FemCap is a new contraceptive device, (Figures 1, 2a, 2b & 2c) designed with a unique delivery system for any vaginal preparation such as gel or cream. These preparations can be applied on the cervical and vaginal sides of the FemCap (Figure 2a & 2b). The aim of this design is to allow for better adherence, coverage, distribution and retention of the preparation for up to 24 hours [4-13].
B.The traditional vaginal applicator, (Figure 3).

C.Vaginal Gel stained with Gentian
Methods
Participants selected for this study were 18 to 40 years old, were not pregnant, and did not desire to be pregnant during the 4-week duration of the study. All subjects were sexually active with no sexually transmitted infections. Thirty participants (75% of whom were sterilized and 25% of whom were on hormonal contraception) signed an informed consent and underwent a complete physical and pelvic exam as well as pap smear and wet mount. Women who have had a STI, vaginitis or abnormal pap smear were excluded.
Twenty women (Group A) were randomly assigned to use the vaginal applicator (Figure 3) and the other 20 women (Group B) were assigned to use the FemCap (Figure 1) to deliver a high viscosity vaginal lubricant stained with Gentian violet dye to enhance its visibility.
After one week of wash over group A then crossed over and used the FemCap and group B used the vaginal applicator for two additional weeks. Participants were instructed to report any side effects while using the vaginal applicator or the FemCap. This facilitated a comparison between the vaginal applicator and the FemCap both to deliver a vaginal lubricant stained with Gentian Violet. This comparison was intended to review the retention and distribution of the stained gel by either device.
We provided participants with hygienic pads to monitor the expulsion of the stained gel and instructed them to bring the pads to the clinic for inspection.
We swabbed each participant’s vagina and introitus for the presence of the stained lubricant at 12 and 24 hours after insertion, as well as photographed the cervix. Finally, the investigators conducted in- depth interviews with each of these 40 women to gain perspective toward our goal to topically treat vaginitis and possibly STIs in future.
Results
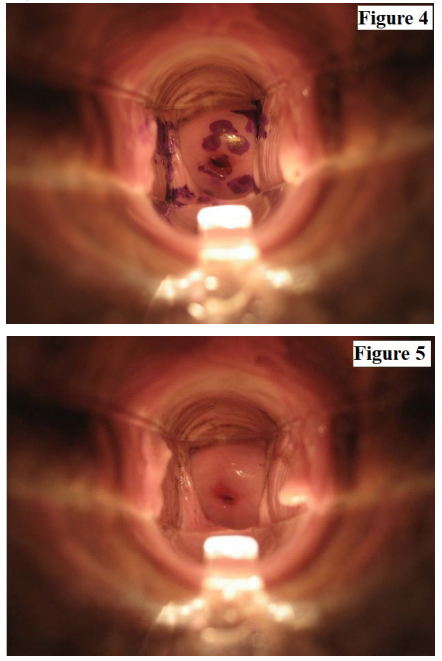
None of the participants reported any significant side effects using either device. Naked eye examination did not show any significant lesions over the cervix or the vagina. Women did not report any leakage while wearing the FemCap. The photograph of their cervix showed the presence of the stain over the cervix 24 hours after application (Figure 4). The women who used the vaginal applicator reported increased leakage of the stained lubricant and there was no presence of the stain 12 hours after the application (Figure 5).
Conclusion
This pilot study demonstrated that the FemCap, when used as a delivery system, has a much better distribution and retention rate than the applicator. This is shown by the photograph of the cervix 24 hours after insertion. This study raised the possibility of topical treatment of common vaginitis and possibly some STIs such as Gonorrhea and Chlamydia. The pilot study did prove the concept that the FemCap can be used as a delivery system for vaginal gels and creams better than the traditional vaginal applicator. The use of the FemCap can also prevent pregnancy and will enhance compliance. The distribution and retention of the therapeutic gel may potentially prevent and treat STIs.
Acknowledgment
This paper was prepared at the request of James D. Fett Editor and Naina K Editorial Assistant, Gynecology & Reproductive Health, we are indebted to the women who consented to participate in this research study, and to Dr. Steven Brody who co-authored and edited this manuscript. No funding was requested or received from any institution. The authors are responsible for the content of this article. This article will be presented at the European Gynecology and Obstetrics Congress February 17-18, 2020 in Paris, France.
References
- Chinmaya Keshari Sahoo, Prakash Kumar Nayak, Deepak Kumar Sarangi, et al. Intra Vaginal Drug Delivery System: An Overview. American Journal of Advanced Drug Delivery. 2013; 1: 43-55.
- Janet G Vail, Jessica A Cohen, Kimberly L Kelly. Improving Topical Microbicide Applicators for Use in Resource-Poor Settings. American Journal of Public 2004; 94: 1089-1092.
- Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005; 73: 1253-1263.
- Carcio H, Clarke Secor M Koeniger-Donohue R. Advanced Health Assessment of Women: Clinical Skills and Procedures Chapter 15 The FemCap. Springer Publishing Company. 2010; 271-278.
- Koeniger-Donohue R. The FemCap A Non-Hormonal Contraceptive. Women’s Health Care. 2006; 5: 79-91.
- Shihata A. The FemCap™, a new contraceptive choice. Eur J Contracept and Reprod Health Care. 1998; 3: 160-166.
- Shoupe D, Kjos S. The Handbook of Contraception, Barrier Contraceptives Chapter 10 Humana Press. 2006; 147-177.
- Shihata A. Microbicides Delivery System. 5th European Congress on Tropical Medicine and International Health. Proceedings Amsterdam. 2007; 131-134.
- Shihata A. Novel Delivery System for Microbicides. Presented in 2007 National HIV Prevention Conference. 2007; 378.
- Shihata New FDA approved woman-controlled, latex- free barrier contraceptive device “FemCap™”. Fertil Steril International Congress Series. 2004; 103: 303-330.
- Shihata Gollub E. Acceptability of a new intravaginal barrier contraceptive device FemCap. Contraception. 1992; 46: 511-519.
- Ariane van der Straten, Nuriye Sahin-Hodoglugil, Kate Clouse, et al. Feasibility and potential acceptability of three cervical barriers among vulnerable young women in Zimbabwe. J Fam Plann Reprod Health Care. 2010; 36: 13-19.
- Shihata A, Brody S. HIV Prevention by Enhancing Compliance of Tenofovir Microbicide. Using A Novel Delivery System. HIV &AIDS Review. 2010; 9: 107-110.