Outcome of Membranous Pulmonary Valve Atresia with Intact Ventricular Septum Treated by Cardiac Catheterization in a Developing Country
Author'(s): Iyad AL-Ammouri MD1*, Abdullah Nimer MD2 , Suzan Naser MD2, Reda Yousef MD2, Mohamed Abu Hani MD2 , Mohammad Abu Fadaleh MD2 and Sahar Karasneh MD1
1Section of Pediatric Cardiology, Department of Pediatrics. School of Medicine, The University of Jordan. Amman 11942, Jordan.
2Department of Pediatrics. School of Medicine, The University of Jordan. Amman 11942, Jordan.
*Correspondence:
Iyad AL-Ammouri, MD, Professor of Pediatric Cardiology,Section of Pediatric Cardiology, Department of Paediatrics, School of Medicine, The University of Jordan, Amman 11942, Jordan, Tel: 0096265353666 ext. 2767; Fax: ++96265300820.
Received: 16 Dec 2022; Accepted: 19 Jan 2023; Published: 25 Jan 2023
Citation: AL-Ammouri I, Nimer A, Naser S, et al. Outcome of Membranous Pulmonary Valve Atresia with Intact Ventricular Septum Treated by Cardiac Catheterization in a Developing Country. Cardiol Vasc Res. 2023; 7(1): 1-6.
Abstract
Background: Pulmonary atresia with intact ventricular septum is a cyanotic heart disease with variable morphological features depending on the degree of right ventricular and tricuspid valve hypoplasia. Currently, the standard management for plate-like, membranous pulmonary atresia includes pulmonary valve perforation using radiofrequency wire and pulmonary valve dilation, with or without ductus arteriosus stenting. Radiofrequency wires are expensive and not available in many developing countries. We report our experience with perforation of the pulmonary valve using coronary wires followed by balloon dilation, with or without ductus arteriosus stenting.
Methods: This is a retrospective review of patients with pulmonary atresia and intact ventricular septum who underwent initial percutaneous treatment at our institution between 2012 and 2020. Patients were followed till March 2022. We describe the baseline anatomy, procedure success and complications, and outcome.
Results: Fifteen patients were treated during the study period. Pulmonary valve perforation using soft end of a hydrophilic-tip coronary wire was successful in 14 patients (93%). Ductus arteriosus stenting was performed in 10 patients. There was one post-procedure mortality due to neonatal sepsis. By the last follow up at median age of 34 months (21-100), twelve patients had 2-ventricular circulation, One with 1.5-ventricle circulation, and one with univentricular circulation.
Conclusions: Perforation of atretic pulmonary valve using soft end of hydrophilic-tip coronary wire for patients with membranous pulmonary atresia and intact ventricular septum is highly successful. Most patients will end up with 2-ventricular circulation.
Keywords
Introduction
Pulmonary atresia with intact ventricular septum (PAIVS) is a rare cyanotic congenital anomaly of the heart which has a range of morphological variations, characterized by absence of communication between the right ventricle and the pulmonary trunk, resulting in a variable degree of hypoplasia of both the tricuspid valve and the right ventricle [1,2]. PAIVS accounts for 3% of all congenital heart defects and is present in 4-8 per 100,000 live births, with male predominance of 1.5:1 [1]. It is thought to be a result of an insult to the pulmonary valve leading to atresia during relatively late embryological development, but the exact mechanism remains unclear [3].
The primary management goal in patients with PAIVS is to achieve biventricular circulation whenever possible. Management algorithms have been suggested, and depend on several factors including the morphology and size of the right ventricle and tricuspid valve, and the anatomy and physiology of coronary circulation [4]. Outcome can range from 2-ventricular, 1.5- ventricular or uni-ventricular circulation [5]. For patients with reasonable right ventricular size, with absence of right ventricle dependant coronary circulation or coronary fistulas, the preferred method is to establish pulmonary circulation through the pulmonary valve by percutaneous procedure [4,6,7]. Often, augmentation of pulmonary blood flow by prolonged use of prostaglandin E-1, or patent ductus arteriosus (PDA) stenting is required for variable period to allow remodelling of the right ventricle [6].
Percutaneous pulmonary valvuloplasty is usually done using radiofrequency wire perforation, followed by balloon dilation of the pulmonary valve [7]. However, radiofrequency wires are expensive and might not be available in low- and middle-income countries. The alternative method is perforation of the atretic valve by coronary guidewire [8,9]. PDA stenting can be done at the same time or at a later occasion if the patient proved to require additional source of pulmonary blood flow.
Since 2012, our percutaneous approach to management of PAIVS involved wire perforation of the pulmonary valve with or without PDA stenting. The purpose of this study is to report the outcome in these patients in terms of survival, type of circulation outcome, procedure success and complications in a middle-income developing country, where radiofrequency wire perforation is not available.
Methods
This study is a retrospective chart review including all patients with PAIVS, who were treated by cardiac catheterization at a single institution during 2012 to 2020. Since 2012, patients who were diagnosed with PAIVS at Jordan University Hospital were assessed for suitability of percutaneous interventions. Patients who had a membranous, plate-like pulmonary atresia, with absence of coronary anomalies by echocardiography were considered for percutaneous interventions. Patients who had long segment atresia of the right ventricular outflow tract, or pulmonary trunk, or those who had demonstrable coronary anomalies by echocardiography were referred for cardiac surgery at a different institution.
We reviewed clinical notes, echocardiographic and angiographic images. Clinical data included the age at diagnosis, weight and length of patient, oxygen saturation at baseline, and whether prostaglandin E-1 infusion was used prior to the procedure. Echocardiographic data at diagnosis included the z-score of both tricuspid valve and pulmonary valve annulus. Angiographic images and reports were reviewed, data included date and type of interventions, complications and outcome. Follow up data included date and age at last follow up, oxygen saturation, dates and types of re-interventions beyond the neonatal period, echocardiographic assessment of tricuspid valve, right ventricular size, residual pulmonary stenosis, and outcome of circulation whether 2-ventricular, 1.5-ventricular, or uni-ventricular physiology.
Categorical data is presented as number (percentage), continuous data is presented as mean (+/- standard deviation), or median (range). P value of less than 0.05 was considered significant. Statistical analysis was done using online graphpad quickcalcs software (www.graphpad.com/quickcalcs, San Diego, USA).
The study was approved by institutional review board at Jordan University Hospital and by research committee of the University of Jordan.
Results
Fifteen patients were diagnosed with membranous pulmonary atresia and intact ventricular septum during the study period of 2012-2020. The median age of diagnosis was 4 days (1-34). The median weight was 3.25 Kg (2.5-3.8). All patients presented with cyanosis, with median oxygen saturation of 75% (68-89). Only one patient had prenatal diagnosis of PAIVS. Echocardiographic assessment of the right ventricle showed that 4 patients had well developed, tripartite ventricle, and 11 with hypoplastic ventricle with reasonable inflow and outflow parts, but small trabeculated body. Median tricuspid valve annulus and Z-score were 8.8mm (6.5-11) and -1.7 (-3.3--0.2), respectively. The median diameter and Z-score of the pulmonary valve annulus were 5.5mm (4-6.5) and -3.3 (-4.8--1.9), respectively. Eleven patients were started on prostaglandin E-1 infusion after making the diagnosis, three patients were planned for intervention soon after the diagnosis and had oxygen saturation >80%, therefore did not need prostaglandins, and one patient was transferred to our institution at the age of 34 days with the diagnosis of PAIVS and patent ductus arteriosus (PDA) with no prostaglandin infusion.
Interventions
All patients underwent attempted pulmonary valve perforation and balloon dilation at a median age of 7 days (2-35). In fourteen patients, the pulmonary valve was perforated successfully using the soft end of a 0.014, hydrophilic tip, complete total occlusion (CTO) coronary wire (Hi-torque Pilot 50, Abbott, Minnesota, USA). Access to the right ventricular outflow tract was achieved using either a 5Fr Judkin right coronary catheter, 4Fr Cobra catheter, or in one case a 5 Fr multipurpose catheter. Once the catheter is in place proximal to the pulmonary valve, the coronary wire was used to perforate the valve with gentle manipulation and rotation of the wire (Figure 1). In one patient, perforation of the valve could not be done due to failure to achieve a proper catheter position in the outflow tract. Once the wire crossed the pulmonary valve, it was manipulated either to the descending aorta through the PDA, or to a branch pulmonary artery. Balloon dilation was usually done in two steps: First with a small-diameter (2-3mm) coronary balloon. Then, a second balloon whose diameter is selected based on the valve annulus (selected diameter = 1.5x measured valve annulus). Median balloon diameter was 8mm (6-10).
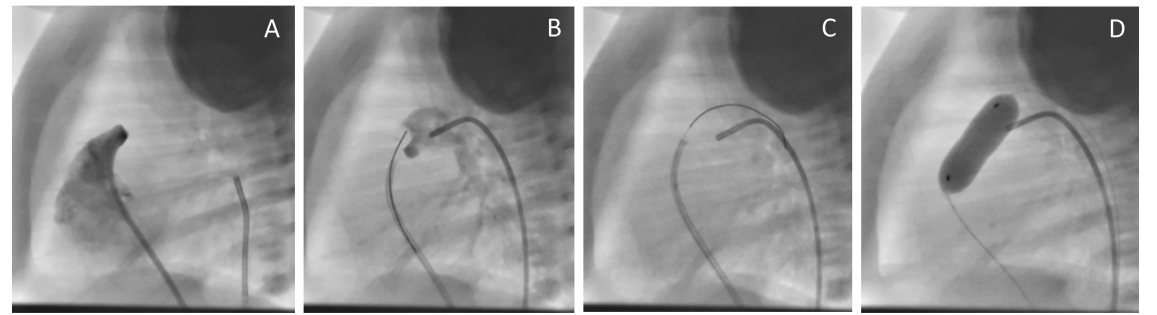
Figure 1: Pulmonary valve perforation and balloon dilation in a patient with pulmonary atresia and intact ventricular septum. A: lateral projection angiography of the right ventricle showing pulmonary atresia. B: A 5 Fr judkin right catheter is placed in the right ventricular outflow tract, with a 0.014 hydrophilic-tipped coronary wire against the pulmonary valve, and a 4 Fr Cobra catheter placed via arterial route into the ductus as a landmark. C: wire perforated through the pulmonary valve and manipulated through the ductus arteriosus. D: balloon dilation of the pulmonary valve.
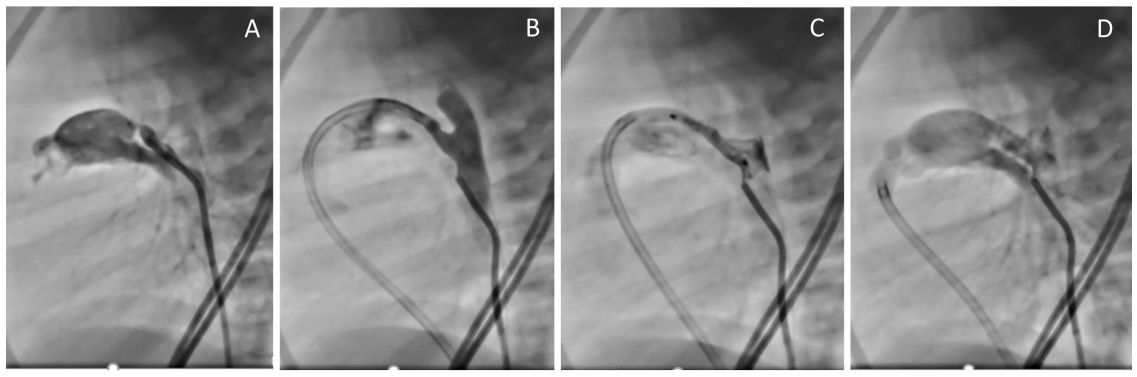
Figure 2: Stenting of ductus arteriosus in a patient with pulmonary atresia and intact ventricular septum following perforation and dilation of the pulmonary valve. A: Aortography via a 4 Fr cobra catheter showing the ductus arteriosus with significant narrowing near the pulmonary end. B: 5Fr Judkin Right guide catheter manipulated over a coronary wire across the pulmonary valve and into the ductus arteriosus. C: an 18 mm long coronary stent is in place covering both ends of the ductus arteriosus before deployment. D: aortogram following stent deployment with a diameter of 3.5 mm showing good flow across the stent.
Total of 10 patients (66%) underwent PDA stenting. Six patients underwent PDA stenting at the time of initial procedure concomitantly with pulmonary valve dilation because the right ventricle was believed to be hypoplastic and additional source of pulmonary blood flow was needed. PDA stenting was done via femoral venous approach following the pulmonary valvuloplasty. The one patient in whom pulmonary valve perforation failed, PDA stenting was done via femoral arterial approach. Among the other 8 patients who underwent balloon dilation alone at the initial procedure, three required PDA stenting 7 days later due to persistence of hypoxia and the need to restart prostaglandin E-1 infusion; two underwent stenting via femoral vein approach, and one via femoral arterial approach. Five patients who underwent initial balloon dilation alone did not require further interventions or prostaglandin infusion. All stents used were balloon expandable coronary stents, with diameter of 3.5-4 mm. Length was determined based on angiographic imaging of the PDA, so that both aortic and pulmonary ends are covered (figure 2). Two patients required an immediate second stent placement, one to cover the aortic end of the PDA because the initial stent was a few millimeters short, the other one to stabilize the initial stent that partially migrated to the pulmonary trunk. All patients with stents were given antiplatelet dose of aspirin for several months, and stopped once the patient has normal oxygen saturation, and with no right to left shunting at the patent foramen ovale, or if the stent had spontaneously occluded.
Complications, Follow up and outcome
Oxygen saturation improved from 75% (±6) before the intervention to 87% (±3.4), p= 0.0001. One patient who had balloon dilation alone died one day after the procedure despite improved oxygen saturation to 88% on room air, later blood culture grew gram negative bacilli. Three patients had wire perforation to the pericardial space, which was immediately recognized, the wire was retracted, repositioned and perforation of the valve was done with no further complications, no pericardial effusion was noted following the procedure in any patient. One patient who had balloon dilation alone was diagnosed with enterococcus infective endocarditis of the pulmonary valve at the age of 2 months, and treated successfully with 6 weeks of intravenous antibiotics. One patient had stent migration to the pulmonary trunk during implantation, which was stabilized with a second stent. Later, the protruding part of the initial stent fractured and the fractured piece resided in the proximal right pulmonary artery, with no effect on the flow. Close follow up was done, lastly at the age of 24 months, which showed stable stent position, with normal flow in both branch pulmonary arteries and trivial PDA flow. One patient developed late stent migration and in-stent stenosis at the aortic end of the PDA, requiring a second stent implantation 5 weeks after the initial procedure, later he underwent superior cavo- pulmonary palliation at the age of 5 months.
Follow up for 14 patients who survived was done for a median duration of 34 months (21-100). Twelve patients had two- ventricular outcome, one patient whose initial TV z score was -2.16 ended up with 1.5 ventricular outcome with a superior cavo- pulmonary connection procedure done at the age of 5 months. His last follow up was at the age of 22 months with oxygen saturation of 97%. One patient with initial TV z score of -2.31 ended up with single ventricular outcome due to severe right ventricular hypoplasia without significant growth despite successful initial procedure, the patient went on to have superior cavo-pulmonary connection procedure with occlusion of the pulmonary valve at the age of 6 months, with last follow up at age of 5.5 years and oxygen saturation of 70% awaiting total cavo-pulmonary palliation. One patient required surgical valvuloplasty of the tricuspid and right ventricular outflow tract augmentation at the age of 8 months. Her last follow up at age 29 months showed two ventricular outcome with mild pulmonary stenosis. One patient required percutaneous atrial septal defect closure at the age of 5 years. Data on patients’ outcomes is summarized in figure 3.
Evaluation of tricuspid valve on the last follow up (for surviving patients with two-ventricular outcome, N=12) showed improved annulus z-score from -1.4 (±0.83) to -0.83 (±0.80), p=0.042. Patients had either mild tricuspid regurgitation (8), or no regurgitation (4). Pulmonary valve evaluation showed median peak gradient measured by echocardiography of 14 mm Hg (9- 29). Seven patients had mild or trivial insufficiency, 5 patients with moderate, and one patient with severe insufficiency.
Regarding stent patency; Four stents had spontaneous occlusion by the time of last follow up, three stents were already removed or ligated surgically during the subsequent surgical interventions (for the patients with uni-ventricular outcome, and 1.5-ventricular outcome, and the patient who needed surgical valvuloplasty of tricuspid and pulmonary valve), and three stents were still patent with trivial insignificant flow.
Discussion
Management of PAIVS has advanced significantly over the last two decades with the introduction of percutaneous interventions of pulmonary valve perforation and dilation, and with PDA stenting. Radiofrequency wires are expensive and not available in our country. However, successful perforation of the pulmonary valve was achieved using a soft end of a hydrophilic-tip coronary wire. Out of 15 patients, this approach was successful in 14 patients (93%), with the one failure being due to inability to achieve catheter position against the pulmonary valve. This approach has been described in literature with reasonable success [8,9]. There is, however a small risk of perforation of the right ventricular infundibulum to the pericardial space with potential complication of pericardial tamponade and death, which has been described particularly with the use of the stiff end of guidewires for perforation [10]. In our series, we used the soft, hydrophilic tip of a 0.014 CTO coronary wire. We did have three incidences of perforation to the pericardial space that was recognized, the wire retracted, and immediate and post-procedure echocardiography showed no pericardial effusion. We do recommend having echocardiography available in the catheterization laboratory while doing such procedure for immediate pericardiocentesis if needed.
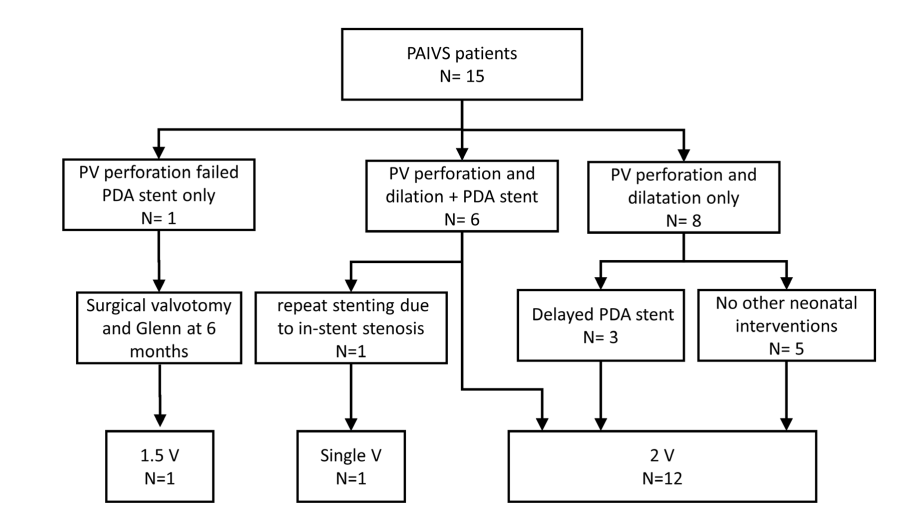
Figure 3: Diagram showing the initial, subsequent procedures, and outcome of 15 patients with pulmonary atresia and intact ventricular septum treated initially by catheter interventions using pulmonary valve perforation and dilatation, with or without ductus arteriosus stenting.
Ductus arteriosus stenting has been a reasonable alternative procedure to surgical aorto-pulmonary shunt in patients with ductus dependant pulmonary circulation with first report of PDA stenting three decades ago [11]. The most commonly used stents are coronary stents, and most common lesions are PAIVS, and pulmonary atresia with VSD among other lesions [12-14]. The purpose of PDA stenting in patients with PAIVS is to augment the pulmonary blood flow while allowing for improved compliance and growth of the hypoplastic right ventricle [13]. PDA stenting is often done during the initial procedure of pulmonary valve perforation and dilation, particularly when there is significant hypoplasia of the tricuspid valve or right ventricle. However, in some patients it may be done a few days or weeks after pulmonary valve dilation [1]. In our series, PDA stenting was done in 10 of the 15 patients (66%), three had delayed stenting after one week of initial procedure. The decision to do primary or delayed stenting depends primarily on the right ventricular size, and we do recommend primary stenting in order to avoid a second procedure if the right ventricle is moderately hypoplastic. However, occasionally PDA stenting may be delayed if it is large at the time of initial intervention, which may lead to instability of the implanted stent. In three of our patients, PDA stenting was delayed, one was due to a large PDA that was not suitable for stenting, so stenting was done when the PDA constricted 7 days later. The other two patients needed delayed PDA stenting due to drop of oxygen saturation requiring restarting prostaglandin E-1.
It has been shown that 2-ventricular outcome in patients with PAIVS should be the goal of management in these patients whenever possible, as it has significantly better survival when compared to single ventricular outcome [15]. Factors that favors 2-ventricular outcome are mainly the size of tricuspid valve and right ventricle, as well as the absence of right ventricle dependent coronary circulation [16]. Even with moderate right ventricular hypoplasia, achievement of 2-ventricular outcome is very likely with perforation and dilation of the pulmonary valve, with or without the need for additional pulmonary blood flow. [17,18] In our series 12 out of 14 survivors (85%) achieved 2-ventricular outcome, while one ended up with 1.5 ventricle, and one with uni-ventricular outcome. In the twelve patients with 2-ventricular outcome, we demonstrated significant growth of tricuspid valve annulus despite the small sample size with increase of tricuspid valve annulus z-score from -1.4 (±0.83) to -0.83 (±0.80), p=0.042. The growth of right ventricle and tricuspid valve has been reported following pulmonary valve perforation and dilatation [17].
Conclusion
Opening of the atretic pulmonary valve by the soft end of a coronary wire is safe and feasible to manage neonates with PAIVS in situations where radiofrequency perforation is not available. PDA stenting is often needed either as a concomitant procedure, or as delayed procedure to augment the pulmonary blood flow. This approach is reasonably successful in achieving 2-ventricular outcome.
Limitations
This study is limited by its retrospective nature, and being a single institution experience with small number of subjects. In addition, the duration of follow up is relatively short, and long term follow up is needed to evaluate the functional status of these patients.
Statements and Declarations
Authors declare no financial, technical, or other assistance received for this work.
Authors declare no conflict of interest associated with this work. Authors ascertain that they had full control of the design of the study, methods used, outcome parameters, analysis of data, and production of the written report.
References
- Chikkabyrappa SM, Loomba RS, Tretter JT. Pulmonary Atresia with an Intact Ventricular Septum: Preoperative Physiology, Imaging, and Management. Semin Cardiothorac Vasc Anesth. 2018; 22: 245-255.
- Anderson RH, Anderson C, Zuberbuhler JR. Further morphologic studies on hearts with pulmonary atresia and intact ventricular septum. Cardiol Young. 1991; 1: 105-113.
- Kutsche LM, Van Mierop LHS. Pulmonary atresia with and without ventricular septal defect: A different etiology and pathogenesis for the atresia in the 2 types?. Am J Cardiol. 1983; 51: 932-935.
- Foker JE, Berry JM, Pyles LA. Treatment algorithm for Pulmonary Atresia with Intact Ventricular Septum. Prog Pediatr Cardiol. 2010; 29: 61-63.
- Lydia K Wright, Jessica H Knight, Amanda S Thomas, et al. Long-term outcomes after intervention for pulmonary atresia with intact ventricular septum. Heart. 2019; 105: 1007-1013.
- Rao PS. Pulmonary Atresia with Intact Ventricular Septum. Curr Treat Options Cardiovasc Med. 2002; 4: 321-336.
- Alwi M, Geetha K, Bilkis AA, et al. Pulmonary Atresia With Intact Ventricular Septum Percutaneous Radiofrequency- Assisted Valvotomy and Balloon Dilation Versus Surgical Valvotomy and Blalock Taussig Shunt. J Am Coll Cardiol. 2000; 35: 468-476.
- Alwi M, Budi RR, Mood MC, et al, Pulmonary atresia with intact septum: the use of Conquest Pro coronary guidewire for perforation of atretic valve and subsequent interventions. Cardiol Young. 2013; 23: 197-202.
- Shweta Bakhru, Shilpa Marathe, Manish Saxena, et al. Transcatheter pulmonary valve perforation using chronic total occlusion wire in pulmonary atresia with intact ventricular septum. Ann Pediatr Cardiol. 2017; 10: 5-10.
- Gabriella Agnoletti, Jean François Piechaud, Philipp Bonhoeffer. et al. Perforation of the Atretic Pulmonary Valve Long-Term Follow-Up. J Am Coll Cardiol. 2003; 41: 1399-1340.
- Gibbs jl, Rothman mt, Rees MR, et al. Stenting of the arterial duct: A new approach to palliation for pulmonary atresia. Br Heart J. 1992; 67: 240-245.
- Mazeni Alwi, Choo KK, Haifa Abdul Latiff, et al. Initial results and medium-term follow-up of stent implantation of patent ductus arteriosus in duct-dependent pulmonary circulation. J Am Coll Cardiol. 2004; 44: 438-445.
- Alwi M. Stenting the patent ductus arteriosus in duct- dependent pulmonary circulation: techniques, complications and follow-up issues. Future Cardiol. 2012; 8: 237-250.
- Valdeomillos E, Jalal Z, Boudjemline Y, Thambo JB. Transcatheter ductus arteriosus stenting in paediatric cardiology: Indications, results and perspectives. Arch Cardiovasc Dis. 2020; 113: 129-141.
- Amrita Sukhavasi, Sara McHugh-Grant, Andrew C Glatz, et al. Pulmonary atresia with intact ventricular septum: Intended strategies. J Thorac Cardiovasc Surg. 2022; 164: 1277-1288.
- Tamer YoldaÅ?, Utku Arman Örün, Vehbi Dogan, et al. Transcatheter radiofrequency pulmonary valve perforation in newborns with pulmonary atresia/intact ventricular septum: Echocardiographic predictors of biventricular circulation. Echocardiography. 2020; 37: 1258-1264.
- Robin H S Chen, Adolphus K T Chau, Pak Cheong Chow, et al. Achieving biventricular circulation in patients with moderate hypoplastic right ventricle in pulmonary atresia intact ventricular septum after transcatheter pulmonary valve perforation. Congenit Heart Dis. 2018; 13; 884-891.
- Shiraz A Maskatia, Christopher J Petit, Curtis D Travers, et al. Echocardiographic parameters associated with biventricular circulation and right ventricular growth following right ventricular decompression in patients with pulmonary atresia and intact ventricular septum: Results from a multicenter study. Congenit Heart Dis. 2018; 13: 892-902.