Pd-L1 Evaluation in Serum and Tumoral Circulating Extracellular Vescicles in Locally Advanced Nsclc After Concomitant Chemoradiation: A Surrogate of Tumor Microenviroment Change from Cold Tumor to Hot Tumor
Author'(s): Grazia Lazzari1*, Tartarone A2, Laurenzana I3, Lamorte D3, Valvano L4, Statuto T4, De Luca L5, D’Auria F5,Caivano A5, Calice G4, Bianculli A6, Tucciariello R6, Lerose R7, Benevento I1, Montagna A1, D’Andrea B1,Castaldo G1 and Angela Solazzo1
1Radiation Oncology Unit, Oncology Reference Center of Basilicata(IRCCS CROB), Rionero in Vulture, Italy.
2Medical Oncology Unit.
3Laboratory of Preclinical and Translational Research.
4Laboratory of Clinical Research and Advanced Diagnostics
5Clinical Pathology Unit
6Physic Unit
7Unit of Hospital Pharmacy
*Correspondence:
Dr. Grazia Lazzari, Radiation Oncology Unit, Oncology Reference Center of Basilicata (IRCCS CROB), Rionero in Vulture, 85028 (PZ) , 1 Padre Pio Street, Italy, Tel: 0972-726740.
Received: 19 Jul 2023; Accepted: 28 Aug 2023; Published: 05 Sep 2023
Citation: Lazzari G, Tartarone A, Laurenzana I, et al. Pd-L1 Evaluation in Serum and Tumoral Circulating Extracellular Vescicles in Locally Advanced Nsclc After Concomitant Chemoradiation: A Surrogate of Tumor Microenviroment Change from Cold Tumor to Hot Tumor. Cancer Sci Res. 2023; 6(1): 1-5.
Abstract
Background: Pacific trial has revolutioned the outcome and the approach in locally advanced (LA) unresectable Non Small Cell Lung Cancer (NSCLC). In 2018 the European Medicine Agency (EMA) approved the use of Durvalumab only in adults whose tumours on biopsy specimen expresses PD-L1 on ≥ 1% of tumour cells despite this cut-off was not provided by the original randomised study. In vitro and in vivo studies have recorded the up regulation of PD-L1 expression in the tumor microenviroment (TME) after chemoradiation regrdless the expression on biopsy. Liquid biopsy could be helpful in detection of circulating PD-L1 in this set. Aim of this observational multicentre prospective study is to assess on liquid biopsy the up-regulation of PD-L1on serum and tumoral circulating extracellular vescicles (cEVS) from patients with LA NSCLC not expressing the PD-L1 on biopsy and treated with concurrent chemoradiation (CCRT).
Methods: Locally advanced NSCLC PD-L1 < 1 % biopsy proven will be enrolled in this study. A blood sample will be taken the day before (T0), 14 days after CCRT (T1) and 1 months later (T2). Patients will be treated with CCRT with platinum-based doublet and 60-66 Gy total radiation dose. Soluble PD-L1 will be analysed by ELISA. PD-L1on cEVs will be isolated from serum and phenotyped by nanoparticle tracking analysis, microscopy and flow cytometry. The statistical significant improvement in PD-L1 expression in serum and cEVs at T0 -T1-T2 will be assessed by t-test for p= 0.05 ; for multiple test p-value will be corrected with FDR.
Keywords
Introduction
Concurrent chemoradiation (CCRT) alone in LA NSCLC has been considered the standard of care for a long time [1,2]. Then PACIFIC trial has added a new milestone in the care of unresectable LA NSCLC providing a consolidation step. The phase III trial assessed the powerful role of Durvalumab, a selective anti- PD-L1 antibody, in the consolidation phase after CCRT. The trial was conducted in patients with LA stage III NSCLC who were not selected based on histology or tumor PD-L1 score [3], Since his pubblication in 2017, Durvalumab consolidation after CCRT still continues to record an unprecedented benefit in OS and PFS [4]. In fact by the last up date, the estimated 5-year OS rates for Durvalumab and placebo have been 42.9% (38.2 to 47.4) versus 33.4% (27.3 to 39.6). Moreover, PFS results have been 33.1%(28.0 to 38.2) versus 19.0% (13.6 to 25.2) [5]. These results were irrespective of PD-L1 status from diagnostic specimen biopsy. While regulatory agencies all over the world have approved Durvalumab as consolidation therapy after CCRT in unselected unresectable stage III NSCLC, in Europe the EMA has limited its use only for patients with PD-L1 expressing tumors ≥ 1% on the basis on an unplanned post hoc analysis of the original trial [6]. Thus in Europe this set of patients after CCRT has no other cure chances [7,8]. This decision has already evoked several concerns by the panel of the international lung cancer experts [9]. Among them, it should be taken into account that the PD-L1 expression in the tumor is not static but is a dynamic outcome of a cross talk between the cancer and immune system [10]. This cross talk is enhanced by radiotherapy as a result of the radiation induced immunogenic cell death [11]. As a confirmation, soluble forms of PD-1 and PD-L1 (sPD-1/ sPD-L1) have been detected in the blood of cancer patients at baseline . Its expression is enhanced by the cytokines cascade occuring with radiation as interleukin-6 (IL-6) and IFN-g [12,13] which have been found to regulate PD- L1 expression. It has clearly been demonstrated in murine models by Dovedi et al how PD-L1 expression is up regulated during RT delivery with a synergistic antitumoral effect when it is delivered concurrently to an immunochekpoint-inhibitor [14]. Moreover Adams has evaluated in NSCLC patients undergone CCRT, the increase of PD-L1 expression in CTCs and a CStC subtype, cancer-associated macrophage-like cells (CAMLs), in response to DNA damage caused by radiotherapy on lung [15]. Further a predictive effect on immonochekpoint inhibitor sensitivity has been also evaluated with immunophenotype, showing the positive prognosticator effect with PDL1 positive an T CD8-positive tumour-infiltrating lymphocytes (TILs) at pre-CRT in patients [16]. PD-L1 is detected in free in solution and in tumoral exosomes or circulant Extracellular Vescicles (cEVs) depending on the sizes (15 nanometers and 10 microns ) which have been found in cancer patients. [17,18]. Exosomes results from double invagination of the plasma membrane and the formation of intracellular multivesicular bodies. Thereafter, they are secreted into the extracellular space and microenvironment by exocytosis carrying all the information from the native tumor cell [19]. Tumor-derived exosomes may also act as regulatory elements that can reprogram the immune microenvironment as in the presenting antigens processes [20]. The isolation of serum cEVs has been validated and is suitable by nanoparticle tracking analysis, microscopy and flow cytometry [21]. Flow cytometry is the most evaluable method to assess the immunophenotype on lymphocytes to check a change from cold to hot tumor more sensitive to immunetherapy. In fact the baseline density of CD8-positive TILs before and after CRT as demonstrated by Shirasawa M et al., seems tobe related to PD-L1 expressiona as a predictive factor for the efficacy of CRT followed by Durvalumab [16]. Given this background, aim of our study is to assess the change of lymphocytes population related to the up-regulation of PD-L1 in serum and cEVs through liquid biopsy as a surrogate of the TME changing after CCRT in LA PD- L1 negative NSCLC patients as a rationale to extend Durvalumab prescription to all elegible patients.
Design
The proposed study is a multicentric observational prospectic study. The flowchart is reported in Figure 1. Patients with biopsy proven locally advanced NSCLC unresectable stage IIIA-IIIB and PD-L1 < 1% will be included. Treatment is the standard care with CCRT as per guidelines, using a platinum based doublet chemotherapy concurrent to lung radiotherapy 60-66 Gy in IMRT o VMAT techinique. At baseline (T0) before CCRT , T1 (2 weeks after the CCRT end) and T2 (1 month later) a fresh blood and serum will be collected for immunophenotype and PD-L1 assessment.
The study has been approved by the local ethycal committee or Unique Regional Ethycal Committee (CEUR 20220039986 N.58/2022).
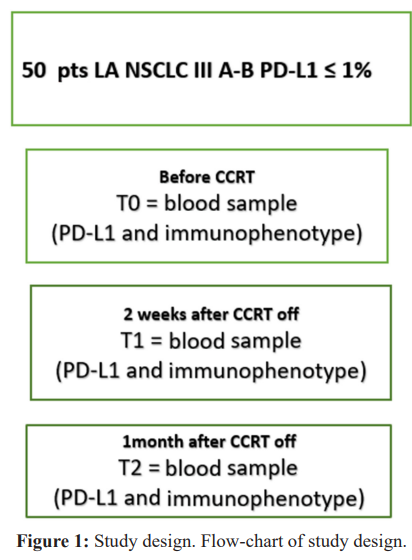
Treatment description
Patients selection
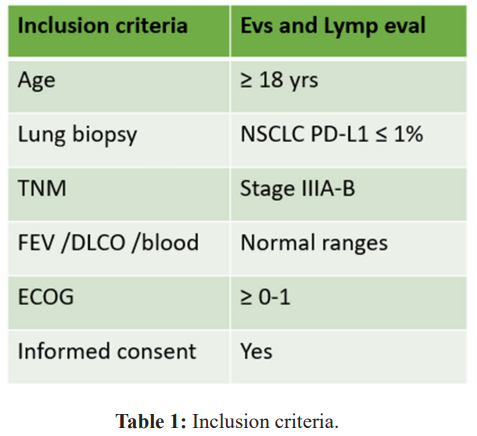
The inclusion and exclusion criteria are listed in Table 1 according to guidelines for advanced unresectable stage IIIA-IIIB NSCLC. Eligible criteria are as follows: age 18 years or older, biopsy- proven, newly diagnosed, primary, locally advanced NSCLC in CT scan and FDG-PET. Pulmonary biopsy will include the PD-L1 status. Patients must be mentally and physically fit for chemotherapy, have an Eastern Cooperative Oncology Group (ECOG) performance score of 0–1 at the time of radiotherapy being available for follow-up, and provide written informed consent. Moreover are required: white blood cell count of 4·0×10â¹ cells per L or higher, platelet count of 100×10â¹ per L or higher, a clinically acceptable haemoglobin level, a creatinine level indicating renal clearance of 50 mL/min or higher, and bilirubin level below 35 μmol/L, FEV1 and DLCO in the normal range. Main exclusion criteria include extensive volumes not elegible to curative doses up 60 Gy; informed consent not provided.
Clinical evaluation
Elegible patients with LA NSCLC will be identified within a multidisciplinary board and then staged with CT scan and FDG- Pet. The day before RT (T0), two weeks and 1 month later CCRT off a fresh blood sample for immunophenotype and serum for PD- L1 will be taken.
Radiation Treatment
Technique and treatment doses
Image guided radiotherapy (IGRT) with VMAT or IMRT will be applied in all patients. A linear accelerator IGRT dedicated with energy of 6MV is required and image verification with digitally reconstructed radiography (DRR) or cone beam computed tomography (CBCT) should be done prior to treatment. The simulation CT scan will be performed in supine position with an immobilization device. The dose will be reported according to the ICRU (International Commission on Radiation Units and Measurements) report 83 [22,23]. The dose to OARs will be assessed according to the dose constraints ICRU [24]. The prescribed dose is 6-66 Gy at the level of PTV with a daily fractionation of 2 Gy over 5 week.
Treatment volumes
According the international guidelines for NSCLC, the clinical target volume (CTV) should include the the primary tumour and relevant regional lymph nodes as defined on imaging plus a 0.5-0.8 margins. Planning target volume (PTV) will correspond to the CTV with a variable margin 0f 0.5 -1 cm at the discretion of the center and image guided RT (IGRT) technique used. The organs at risk (OARs) will be both lungs, spinal cord, esophagus, heart according ESTRO guidelines [25].
Concurrent Chemotherapy
A doublet platinum -based will be delivered . The choice of schedule (vinorelbine, gemcitabine, taxanes) is at the discretion of the individual center in relation to clinical and pathologic features [26].
Immunophenotype and PD-L1 assessment
Blood samples will be collected to assess the impact on adaptive and innate immunity cells and PD-L1 expression at baseline and thereafter the CCRT. Immunophenotype characterization will be runned by cytophluorimetry assessing total T lymphocytes (CD3+), T helper (CD3+ CD4+), T cytotoxics (CD3+ CD8+), T regolators (Tregs: CD4+ CD25+ CD127low ), T double negative (DNT: CD3+ CD4- CD8- CD16- CD56), T double positives (DPT: CD3+ CD4+ CD8+), T natural killer: CD3± CD16+ CD56+) and B (CD19+) with fluorochromes monoclonal antibodies. The isolation of extracellular vescicles (cEVs), the analysis of size distribution and concentration by nano-particle tracking analysis will follow. Thereafter the quantification and the PD-L1 phenotyping of cEVs by flow cytometer will be performed togheter to the quantification of soluble form of PD-L1 by ELISA assay. A statistical avalysis of results concerning the PDL-1 expression soluble and in the cEVs , immunophenotype correlation and clinical response will be evaluated. Thus cytofluorometry and soluble PD-L1 and ECVs will be analysed. The increase of expression of PD-L1 in serum and cEVs from baseline at T1 and T2 will be considered as a surrogate outcome of change from cold to hot tumor [27,28].
Endpoints
The primary endpoint of this study is to evaluate in patients with LA NSCLC PD-L1 after CCRT the dynamic change of PD-L1 expression from cold tumor to hot tumor through liquid biopsy. The secondary endpoint is to relate the PD-L1 expression with immunophenotype as a predictive factor to response to immunotherapy.
Statistics
Sample size
Statistic significance is fixed for p < 0.05. Logistic regression model will be applied to relate the change fron cole tumor to hot tumor and the lymphocyte population. A sample size of 50 patients in 3 years is required . Data will be collected and analysed by t-test. Data analysis will last 5 years
Data collection procedure
Data from each center will be collected in electronic case report forms (CRFs) and transfered into a single cloud-based database. Subsequently, the aggregated data will be processed by the promoter center.
Planned timeline
It is scheduled as follows: 0–3 months: project organization; 18- 36 months: patient enrolment; 48-60 months: laboratory work assessment statistical analysis and publication of data about primary end-point.
Ethics committee approval for ongoing research
The protocol has been written according to the principles of good clinical practice (GCP). This study is conducted in accordance with the most recent version of the Declaration of Helsinki and with the Italian laws and regulations. The study protocol was approved by the ethics committee of promoter center (ethics committee identifier code CEUR). Approval by the respective ethics committee relevant to each site will be collected before opening new sites. Written informed consent, signed and personally dated is obtained from each patient before inclusion in the study.
References
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010; 28: 2181-2190.
- Curran WJ, Paulus R, Langer CJ, et Sequential vs Concurrent Chemoradiation for Stage III Non–Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. J Natl Cancer Inst. 2011; 103: 1452-1460.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung N Engl J Med. 2017; 377: 1919-1929.
- Antonia SJ, Villegas A, Daniel D, et Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018; 379: 2342-2350.
- Spigel DR, Faivre-Finn C, Gray JA, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung JCO. 2022; 40: 1301-1311.
- Faehling M, Schumman C, Christopoulos P, et Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non-small cell lung cancer (NSCLC): Real-world data on survival and safety from the German expanded-access program (EAP). Lung Cancer. 2020; 114-122.
- European Agency Medicines: Imfizi: EPAR – Product information. 2018. 7.
- European Medicines Agency: Imfizi: EPAR – Public Assessment Report, EMA/CHMP/548232/2018, 2018.
- Peters S, Dafni U, Boyer M, et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann of Oncol. 2019; 30: 161-165.
- Weichselbaum RR, Liang H, Deng L, et al. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rew Clin Oncol. 2017; 14: 373.
- Luhillieur C, Rudqvist NP, Yamazaki T, et al. Radiotherapy- exposed CD8+ and CD4+ neoantigens enhance tumor J Clin Invest. 2021; 131: e138740.
- Fang W, Zhang J, Hong S, et al. EBV-Driven LMP1 and IFN-g Up-Regulate PD-L1 in Nasopharyngeal Carcinoma: Implications for Oncotargeted Therapy. Oncotarget. 2014; 5: 12189-202.
- Xu L, Chen X, Shen M, et Inhibition of IL-6- JAK/ StatSignaling in Castration-Resistant Prostate Cancer Cells Enhances the NK Cell-Mediated Cytotoxicity via Alteration of PD-L1/NKG2D Ligand Levels. Mol Oncol. 2018; 12: 269- 286.
- Dovedi SJ, Illidge The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunol. 2015; 4: e1016709.
- Adams D, Adams KD, He J, et al. Sequential Tracking of PD- L1 Expression and RAD50 Induction in Circulating Tumor and Stromal Cells of Lung Cancer Patients Undergoing Clin Cancer Res. 2017; 23: 5948-5958.
- Shirasawa M, Yoshida T, Imabayashi T, et al. Baseline PD- L1 expression and tumour-infiltrated lymphocyte status predict the efficacy of durvalumab consolidation therapy after chemoradiotherapy in unresectable locally advanced patients with non-small-cell lung cancer. Eur J Cancer. 2022; 162: 1-10.
- Kalluri R, LeBleu The Biology, Function, and Biomedical Applications of Exosomes. Science. 2020; 367: 6977.
- Li S, Yi M, Dong B, et al. The Role of Exosomes in Liquid Biopsy for Cancer Diagnosis and Prognosis Prediction. Int J 2021; 148: 2640-51.
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019; 88: 487-514.
- Yan W, Jiang Immune Cell-Derived Exosomes in the Cancer-Immunity Cycle. Trends Cancer. 2020; 6: 506-517.
- Laurenzana I, Trino S, Lamorte D, et al. Analysis of Amount, Size, Protein Phenotype and Molecular Content of Circulating Extracellular Vesicles Identifies New Biomarkers in Multiple Int J Nanomedicine. 2021; 7; 16: 3141-3160.
- The International Commission on Radiation Units and Journal of the ICRU 2010; 10: NP.2-NP.
- ICRU Report 62, Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU 50) – ICRUn.d.https://www.icru.org/report/prescribing-recording- and-reporting-photon-beam-therapy-report-62/(accessed December 8, 2021).
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010; 76: S10-9.
- Nestle U, De Ruysscher D, Ricardi U, et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung Radiother Oncol. 2018; 127: 1-5.
- Higgins KA, Puri S, Gray Systemic and Radiation Therapy Approaches for Locally Advanced Non-Small-Cell Lung Cancer JCO. 2022; 20; 40: 576-585.
- McKinnon K. Flow Cytometry: An Overview. Curr Protoc 2018; 21; 120: 5.1.1-5.1.11.
- Konoshenko MY, Lekchnov EA, Vlassov AV, et al. Isolation of extracellular vesicles: general methodologies and latest Biomed Res Int. 2018; 8545347.