Preliminary Assessment of a Newly Designated Predictive Index for Relapse in a Treated Cohort of IgG4 -RD Egyptian Patients
Author'(s):Hany El-Saadany*
Kobri El-Kobba medical complex, Cairo, Egypt.
*Correspondence:
Hany El-Saadany (MD), Kobri El-Kobba medical complex, Cairo, Egypt, E-mail: hanyelsadany@yahoo.com.
Received: 12 May 2018; Accepted: 18 June 2018
Citation: Hany El-Saadany. Preliminary Assessment of a Newly Designated Predictive Index for Relapse in a Treated Cohort of IgG4 -RD Egyptian Patients. Clin Immunol Res. 2018; 2(1): 1-4.
Abstract
Background: IgG4 related disease (IgG4-RD) is systemic fibroinflammatory condition characterized by remissions and relapses. Glucocorticoids and rituximab are used for inducing remission. The duration of remission variable and the current predictors of relapse are insufficient and depend mainly on laboratory measures ignoring the clinical picture of the patient.
Objectives: At our center, we adopt 6 measures in a newly designated index to predict any relapse of the disease after treatment of IgG4-patient. The Objective of this prospective study is a preliminary assessment of this index in predicting the disease relapse.
Methods: In a prospective cohort study, during the period of June 2015 till June 2017. 25 Egyptian patients full filing the clinicopathological criteria for diagnosis of IgG4-RD in Kobri El-Kobba medical military complex, are included in the study. Of them, 21 patients are treated by glucocorticoids and 4 by rituximab 2 doses of 1gm with 15 days in between. At our center, we adopt using 6 measures in a newly designated prediction index for relapse extending the benefits of the current predictors of relapse which are mainly laboratory. The new index, which is named for simplicity (Saadany index) after the surname of the author, consists of a very trusted measure based on a clinical bases which is IgG4 responder index (IgG4- RI) and a questionnaire of the patient assessing the well-being and the daily activity in addition to the laboratory predictor parameters of elevated IgE titer, circulating eosinophils, circulating plasma blasts by flow cytometry gated to CD138, CD38, CD20, IgG4 titer. Estimation of the score is as follows,4 points to IgG4-RI, and 1 point to any other elevated element of the other parameters than the baseline before treatment to make the sum at its maximum 9 points. Measurements are done after 1 month of the end of glucocorticoid treatment and 6 months of the end of rituximab treatment respectively and then once quarterly for the rest of 2 years.
Results: 17 patients of the 21 (80.9%) treated with glucocorticoids that had a score less than 3/9 didn’t relapse. 4 patients (19%) who had a score more than 3/9 developed a relapse. 2 of them (50%) which had a score 6,7 respectively developed multisystem relapse. 4 patients of 25 included in the study are treated with rituximab, 3 of them which had a score less than 3/9 didn’t relapse and the other (25%) who had a score 5/9 relapsed.
Conclusion: Up to our experience at our center, the use of this simple, easy applicable, mixed clinical and investigative measurement index, predicts well the possibility of relapse after treatment of a cohort of IgG4-RD patients either by glucocorticoids or rituximab and the higher scores are associated with a multisystem relapse. Further research probably a large multicenter prospective study is recommended for more accurate evaluation.
Keywords
Introduction
IgG4-related disease (IgG4-RD) is a systemic fibroinflammatory condition of unknown etiology that can affect nearly any anatomical site [1,2]. Untreated disease can lead to perminent organ damage and life threatening complications [3,4]. The gold standard for the diagnosis is the characteristic histopathology with a significant infiltrate of IgG4+ plasma cells. Both glucocorticoids and rituximab are effective in inducing remission in IgG4-RD [5-7]. IgG4-RD often follows an unpredictable relapsing pattern, and the optimal strategy for maintenance therapy (e.g low dose glucocorticoids), retreatment with rituximab, or other is unclear [8]. A better understanding of relapse predictors is important for optimal disease management. We examined predictors of disease relapse in 25 IgG4-RD Egyptian patients with different organ involvement most of them were treated with glucocorticoids and some with rituximab.
Methods
Cohort overview and patients selection:
25 IgG4-RD patients who were biopsy proven and started treatment either by glucocorticoids or rituximab are included in the study (n=25).The extent of organ involvement was determined by review of the patient's history, clinical examination radiological and laboratory investigations as well as biopsies. Multiorgan disease in our study indicates more than one organ. Assessment of organ function determines the extent of possible damage either radiological or biochemical. Activity of the disease was assessed by IgG4-RD responder index (RI) [9,10].
Pathology methods
All patients had at least one positive tissue biopsy including dense lymphoplasmacytic infiltrate, storiform fibrosis, and obliterative phlebitis [9]. At least 2 of these features were present in all cases. We considered >10 IgG4+ plasma cells per high power field and an IgG4+/IgG+ ratio >0.040 to be supportive to the diagnosis with clinical correlation. Serum IgG4 assays, circulating plasma blasts gated to CD 138, CD38, CD24, CD27, CD19 before and after treatment by flow cytometry of the peripheral blood were measured (1 month after glucocorticoids and 6 months after rituximab respectively.
Rituximab administration
The patients treated by rituximab are selected either due to incomplete response to glucocorticoids or due to great morbidity [eg IgG4 related sclerosing mesenteritis [2] or IgG4 sclerosing cholangitis [2] received 100 mg hydydrocortisone plus 25 mg diphenhydramine 30 minutes before administration of 1gm rituximab twice with 15 days apart.
Assessment of response to therapy
Clinical responses were defined as a decline in the IgG4-RD RI of at least two points over base line [8], with appropriate follow up by radiological and laboratory investigations. Relapse was defined by the new development or return of clinical manifestations or laboratory investigations favoring IgG4 activity. We didn't rely upon the titer of IgG4 as an indicator of relapse.
Potential predictors of disease relapse
Previous studies have suggested that certain variables are potential relapse predictors like serological and flow cytometric parameters [11-13]. We considered relapse when a disease activity registered just once after remission induction.
Statistical analysis
Statistical differences were analyzed by Student’s t test, the appropriate non parametric test, or Fisher’s exact test, depending on the variable. P < 0.05 was considered significant for all statistical significance testing.
Results
Features of the 21 patients treated with glucocorticoids
These are shown in table 1. The majority of the patients had one organ involvement namely salivary glands, orbital structures, pancreases, biliary system, thyroid gland and mesenteric fat.
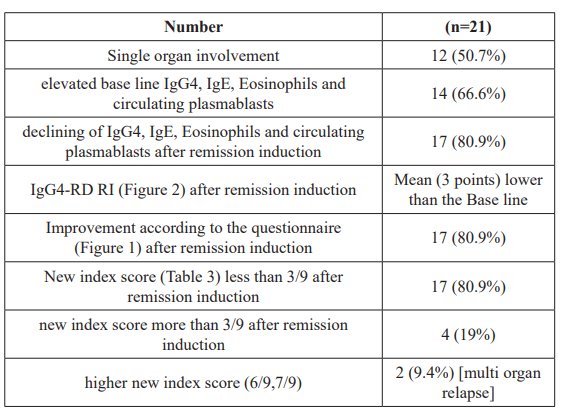
The base line levels of IgG, IgE eosinophils and circulating plasmablasts are elevated in 66.6% of patients at time of the diagnosis and decreased in 80.9% of treated patients adter remissiin induction. The responder index was improved by a mean of 3 points after remission induction by glucocorticoids. Also, 80.9% of treated patients after remission induction responded positively for improving by the questionnaire. The same percentage 80.9%, interristingly, had a new index score >3/9 of which; the higher scores 6/9,7/9 were associated with multiorgan relapse.
In group (2) treated by rituximab ,single organ involvement observed in 50% of patients. There was an elevation in IgG4, IgE, eosinophils and circulating plasmablasts at the time of diagnosis in the majority of patients (75%), on the other hand,there was a decline in them after remission induction by rituximab in the majority also (75%). The same finding observed above were registered in this group in terms of improvements in both the IgG4-RD RI and the patient's questionnaire. Also,the new index score went hand in hand with the above mentioned regarding the cases in remission or the relapsed respectively.

Figure 1: The Patient’s Questionnaire:
The questionnair consists of 4 questions each positive answer by yes is estimated to equal 1/4 of the point.

Figure 2: The IgG4-RD RI.
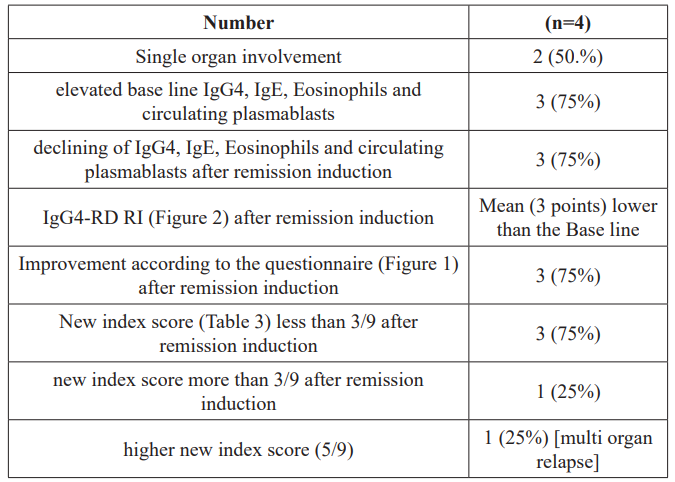
Table 2: Features of patients treated with rituximab.
The index consists of 6 items as shown in table 3 each item had score the first one which is the IgG4-RD responder index it's score is 4 points giving a special importance to the clinical data. Each of the other items has a score 1 point to make the total score as 9 points. We considered the score >3/9 is an indication of relapse.
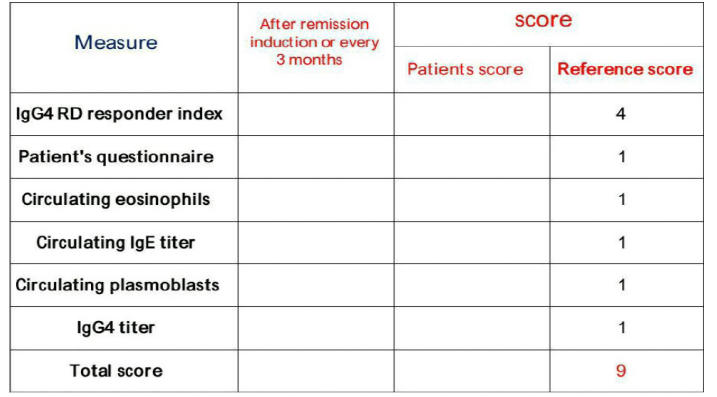
Table 3: The newly desighnated index for prediction of relapse in IgG4- RD.
Discussion
This study concentrated on possible predictive factors other than those described before namely, elevated circulating eosinophils, circulating plasma blasts, IgG4 and IgE titers [11,12,14].
Our center included the IgG4-RD RI as a good clinical measuring index to be incorporated in the new predictive index taking the opportunity to express the clinical response to therapy either to the glucocorticoids or to rituximab [15]. Also, a patient’s questionnaire was developed of four questions designed to express the subjective status of the patient; exploring his own opinion about his well- being. The above two measures were added to the previously mentioned four laboratory measures for prediction of disease relapse after remission induction (1 month after glucocorticoids and 6 months after rituximab) and quarterly for the rest of 2 years. There was a great similarity between the 2 groups regarding the new predictive index as well as the individual items within the index. The elevated circulating plasmablasts, eosinophils, IgG4 and IgE titers are significantly high p<0.05 in the two groups before treatment as shown in table (1) supporting the previous findings that these variables are high at the time of diagnosis (base line) [11,12,16]. Also, the decline in these parameters after remission induction (80.9%) in group 1 and (75%) in group 2 goes hand in hand with the observation by some authors that they are good measures for the success of treatment, on the other hand ,rise of these measures in 19% of group 1 and 25% of group 2 who were relapsed supported the observation that they are good predictors of relapse [11,12]. Regarding the IgG4-RD RI results all support the previous findings detected before by some authors [17]. The patient’s questionnaire, up to our view, aids much to assess the patient’s well-being according to his opinion The incorporation of the 6 parameters in one newly designated index at our center; expands the benefits of combining the clinical assessment, subjective view of the patient in addition to a measurable laboratory parameters which refine the judgments of the relapse. A score >3/9 is considered good predictor of relapse and expresses the relapsed cases in the 2 groups. Interestingly, the higher scores, 5/9, 6/9, 7/9 were in parallel to multiorgan relapse in the 2 groups.
Conclusion
Up to our experience at our center, the use of this simple, easy applicable, mixed clinical and investigative measurement index, predicts well the possibility of relapse after treatment of a cohort of IgG4-RD patients either by glucocorticoids or rituximab and the higher scores are associated with a multisystem relapse. Further research probably a large multicenter prospective study is recommended for more accurate evaluation.
References
- Terumi Kamisawa, Yoh Zen, Shiv Pillai, et IgG4-RD. Lancet. 2014; 385: 1460-1471.
- John Stone, Yoh Zen, Vikram Deshpande, et al. IgG4-RD. N Engl J Med. 2012; 366: 539-551.
- Stone Case records of the Massachusetts general hospital. Case 38-2012. N Engl J Med. 2012; 363: 2335-2346.
- Tong AK, Tan SY, Go YY, et Cardiac structural abnormalities associated with IgG4 related coronary periarteritis and inflammation revealed by multimodality imaging. Can J cardiol. 2014; 30: e15-e17.
- Khosroshahi A, Carruthers MN, Deshpande V, et Rituximab for the treatment of IgG4-RD. Lessons from the consequative patients. Medicine. 2012; 91: 57-66.
- Hart PA, Topazian MD, Witzig TE, et Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab. The Mayo clinic experience. Gut. 2013; 62: 1607-1615.
- khosroshahi A, Stone Treatment approaches to related systemic disease Curr opin Rheumatol. 2011; 23: 67-71.
- Yamamoto M, Takahashi H, Ishigami K, et Relapse patterns in IgG4-RD. Ann Rheum Dis. 2012; 71: 1755.
- Mollie N. Carruthers, John H. Stone, Vikram Deshpande, et Development of an IgG4-RD. Ann Responder index .Int J Rheumatol. 2012; 259408.
- Carruthers MN, Khosroshahi A, Augustin T, et The diagnostic utility of serum IgG4 concentration in IgG4-RD. Ann Rheum Dis. 2014; 74: 14-18.
- Mattoo De novo oligoclonal expantion of circulating plasma blasts in active and relapsing IgG4 -RD. J Allergy clin immunol. 2014; 134: 679-687.
- Wallace ZS, Mattoo H, Carruthers M, et plasma blasts as a biomarker for IgG4-RD independent of serum IgG4 concentrations. Ann Rheum Dis. 2014; 74: 190-195.
- Yamamoto M, Nojima M, Takahashi H, et al. Identification of relapse predictors in IgG4-RD using multivariate analysis of clinical data at the first visit and initial treatment.Rheumatology. 2014; 54: 45-49.
- El-Saadany HA. Case series Association between elevated circulating plasma blasts and their gated flocytometric picture in a cohort of diversity of clinical presentations of IgG4-RD Je Clin Cell Immunol. 2017; 8.
- Wallace ZS, Mattoo H, Mahajan VS, et Predictors of disesse relapse in IgG4-RD following rituximab. Rheumatology. 2016; 55: 1000-1008.
- Della Torre E, Mattoo H, Mahajan VS, et al. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-RD. Allergy. 2014; 69: 269-272.
- Consensus statement of the pathology of IgG4-related disease. Mod pathol. 2012; 25: 1181-1191.