Prevalence of Diabetes Mellitus among Tuberculosis patients in Bangladesh
Author(s): Abdullah Al Mamun, Md1,2, Adil Maqbool3, Farzana Sohael4, Arifur Rahman, Md5, Majedul Islam, Md2,6, Takeru Shima7, Nobutake Shimojo6, Satoru Kawano8, Naoto Yamaguchi8, Masao Moroi9, and Subrina Jesmin2,9*
1Combined Military Hospital and Army Medical College, Bogura, Rajshahi, Bangladesh.
2Health & Disease Research Centre for Rural Peoples (HDRCRP), Mohammadpur, Dhaka-1207, Bangladesh.
3Allama Iqbal Medical College, University of Health Sciences (UHS) Lahore, Pakistan.
4Department of Gynecology, Dhaka Medical College, Dhaka, Bangladesh.
5Shaheed Ziaur Rahman Medical College, Bogura, Bangladesh.
6Department of Emergency and Critical Care Medicine, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki, Japan.
7Department of Health and Physical Education, Cooperative Faculty of Education, Gunma University, Gunma, Japan.
8Ibaraki Prefectural University of Health Sciences, Ami, Japan.
9Faculty of Medicine, Toho University Graduate School of Medicine, Tokyo, Japan.
*Correspondence:
Subrina Jesmin, MD, Ph.D., Faculty of Medicine, TOHO University Graduate School of Medicine, 5-21-16 Omori-nishi, Ota-ku, Tokyo 143-8540, Tokyo, Japan, Tel: +81-90-6198-2673.
Received: 10 Jan 2022 Accepted: 08 Feb 2022 Published: 12 Feb 2022
Citation: Abdullah Al Mamun, Maqbool A, Sohael F, et al. Prevalence of Diabetes Mellitus among Tuberculosis patients in Bangladesh. Diabetes Complications. 2022; 6(1); 1-8.
Abstract
Aim: Tuberculosis (TB) and diabetes mellitus continue to be significant public health problems. Diabetes screening of tuberculosis patients will provide early case diagnosis, improved diabetes control, and improved tuberculosis treatment result. The purpose of this study was to ascertain the prevalence and risk factors for diabetes in tuberculosis patients, as well as their influence on the treatment result of tuberculosis.
Methods: This cross-sectional study was conducted at Bangladesh's randomly chosen peripheral health institutions. All TB patients above the age of 18 years were checked for diabetes. Age, gender, family history, smoking, and BMI were all considered as risk factors.
Results: Out of 165 participants, diabetes was shown to be prevalent in 35% of TB patients. Diabetes was related to advanced age and type of tuberculosis; its origin (pulmonary or extra pulmonary) and socioeconomic level were found to be significant.
Conclusion: Diabetes is a common comorbidity among tuberculosis patients. Over the age of 50 years, the risk of this twin morbidity increases significantly. Screening tuberculosis patients for fasting blood sugar values will aid in the early diagnosis of diabetes and will lead to a better prognosis.
Keywords
Introduction
Now, non-communicable diseases (NCDs) are a global issue that overwhelmingly affects low- and middle-income countries (LMICs), where the infectious disease burden is often large. In 1990, the NCDs prevalence was estimated to be 47% in LMICs, but it is predicted to outnumber communicable diseases by 2030 [1]. Industrialization and urbanization advancements have resulted in changes in lifestyles, most notably in eating preferences, resulting in a higher prevalence of obesity and type II diabetes mellitus (DM). There are about 422 million people worldwide who have diabetes, with approximately 80% of cases occurring in low- and middle-income countries (LMICs) [2,3], where concurrently communicable diseases such as tuberculosis (TB) are often endemic [4]. Type 2 diabetes accounts for about 90% of diabetes cases, with an even greater incidence in urban and elderly communities [5].
Tuberculosis (TB) is the world's leading infectious disease killer. South Asia, comprised of eight LMICs, namely Bangladesh, Afghanistan, Bhutan, India, Pakistan, Maldives, Nepal, and Sri Lanka, accounts for approximately 44% of global tuberculosis infections [6] and a significant rate of tuberculosis mortality (681, 975 deaths), accounting for 38% of the global burden [7]. The high burden of tuberculosis is complicated further by the increasing prevalence of multiple risk factors in South Asia, including kidney disease, acquired immunodeficiency syndrome, malnutrition, and diabetes, and by health system- and patient-related factors such as availability diagnosis, and treatment completion [8].
Cardio metabolic diseases, especially diabetes, have developed into a major health issue in South Asian countries, with diabetes prevalence estimated to increase by more than 151% between 2000 and 2020 [9]. Additionally, some findings show that South Asians are more likely to experience cardio metabolic disorders such as diabetes than other ethnic groups [10].
Numerous studies have identified a bidirectional relationship between diabetes and tuberculosis. Diabetes is hypothesized to worsen the therapeutic path in tuberculosis care. Additionally, TB impairs diabetics' glycemic function [11]. A possible explanation for the TB connection is that diabetics have a compromised host defense. The literature review demonstrates that TB in DM patients is associated with various risk factors, including sociodemographic characteristics, family history of tuberculosis, tobacco use, and type of tuberculosis [12-14].
Currently, literature on diabetes mellitus (DM) in tuberculosis (TB) patients in Bangladesh is limited. In this context, the current research sought to determine the prevalence of diabetes and its risk factors among tuberculosis (TB) patients undergoing therapy.
Materials and Methods
In this cross-sectional study, 165 patients, a new or existing TB cases ≥18 year, including pulmonary, extra pulmonary and both cases, were participated. This study was conducted in Bogura sadar upozila (sub district), Bogura, Bangladesh. They were included taking treatment under hospital and home currently cured recurrence or abandonment, and those visiting for taking ant tubercular medicines.
On the first day, patients were told about the study's goal, a participant information sheet was distributed, and informed written permission was acquired. We questioned all willing participants using a pretested questionnaire. The questionnaire included questions about diabetes risk factors such as age, gender, alcohol consumption, family history, and smoking. We determined current tobacco or alcohol use, the number of cigarettes smoked per day, and the number of years smoked. Details on the timing of diagnosis and therapy were gathered for individuals who had previously been diagnosed with diabetes.
In addition, we measured the participants' height, weight, and waist circumference. The weight was determined to the closest 0.5 kg using a weighing scale and standard minimum apparel. To the closest 0.5 cm, height was measured vertically with the heel, buttocks, and occiput against the wall. The waist circumference (WC) was determined at the level of the iliac crest's greatest point. The threshold for WC was set at 90 cm for men and 80 cm for women to assess abdominal obesity indicative of high risk. The body mass index (BMI) was computed using the formula BMI = (weight in kilograms)/ (height in meters squared)2. The BMI was utilized to classify the participants' nutritional status according to the established cutoffs for Asians (Normal BMI: 18.0-22.9 kg/m2; Overweight: 23.0-24.9 kg/m2; Obesity: >25 kg/m2) [15].
All the selected patients were asked to report the next day following an overnight fast. The patient's fasting blood glucose and Glucose challenge test (GCT) level was determined using a standardized glucometer. Patients with abnormal fasting blood sugar readings or the presence of two or more risk factors were referred to and treated by the medical officer. Blood pressure was taken in a sitting posture from the right arm using a conventional mercury manometer. Each patient's blood pressure was tested twice: first after 5 minutes of relaxation and again after a 15-minute interval; the mean of the two readings was obtained and recorded.
Ethics and informed consent
Prior to patient enrollment, the review board of our institution’s research committee (BOG.DM-TB-22) authorized the study. Each participant received all research information as well as assurances of confidentiality. The research only included individuals who gave their permission.
Statistical analysis
The paired student's t-test and the Mann-Whitney test for normal and skewed continuous variables, respectively, were used to compare all demographic and clinical features between the two groups (non-diabetic and diabetic) (IBM SPSS Statistics, version 20). We utilized the chi-square test for all categorical comparisons between the two groups (STATA, version 10). We used Microsoft Excel to compute the numbers in the graph (Figures 1, 3, 4 and 5). Data are provided as Mean S.D. or percentage (percent) in Tables 1-3, and as Odds ratio (OR) and 95 percent confidence interval in Table 4. Data are given as percentages in Figures 1 and 2. (Percent). For all parameters in the present investigation, a P-value of 0.05 was deemed statistically significant. The results in Figures. 3-5 are shown as Mean.
Results
There were 165 tuberculosis patients in all, of whom 58 (35%) were classified as diabetic, and 107 (65%) were determined to be non-diabetic. The participants' mean age was 45.2 ± 16.5 years. Mean level of body mass index (BMI) 19.5 ± 3.0 kg/m2. Mean level of waist circumference 75.1 ± 8.3 cm, regarding the age (p=0.005), smoking habit (p=0.010), tobacco consumption (p=0.009), fasting blood sugar (p<0.001), glucose challenge test (p<0.001), Fruits servings/week (p=0.031), vegetable servings/week (p=0.006) and types of tuberculosis (p=0.029) all were statistically significant in both of the groups. Talking about gender distribution, of the total number of tuberculosis patients diagnosed with type 2 diabetes, 41 (70.7%) were male, and 17 (29.3%) were female Participants over the age of 50 and male tuberculosis patients were shown to be significantly more likely to have diabetes than female and younger patients. When compared to the non-diabetic group, diabetics have a greater family history of diabetes and TB. In this study, there was no significant link between BMI or waist circumference and diabetes. People with tuberculosis and diabetes are severely underweight and lose weight at a faster rate. The majority of the cases are people who have just been diagnosed with TB (94.8%). The majority of patients (n = 123; 74.3%) had pulmonary tuberculosis as compared to extra pulmonary TB (n = 37; 22.4%), whereas 3.3 percent (n=5) had both pulmonary and extra pulmonary tuberculosis. Around 60.9% received treatment in a hospital setting, whereas 39.1% received treatment at home following diagnosis. A 96.8% of them were cured, 2.6% experienced recurrence, and 0.7% experienced abandonment (Table 1).
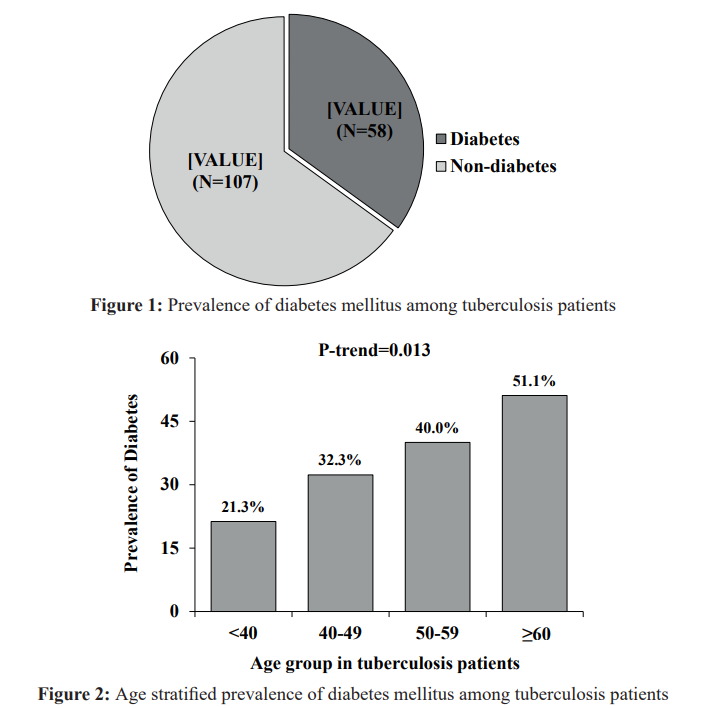
Among TB patients 35% were diabetic subjects and 65% were non-diabetic subjects (Figure 1).
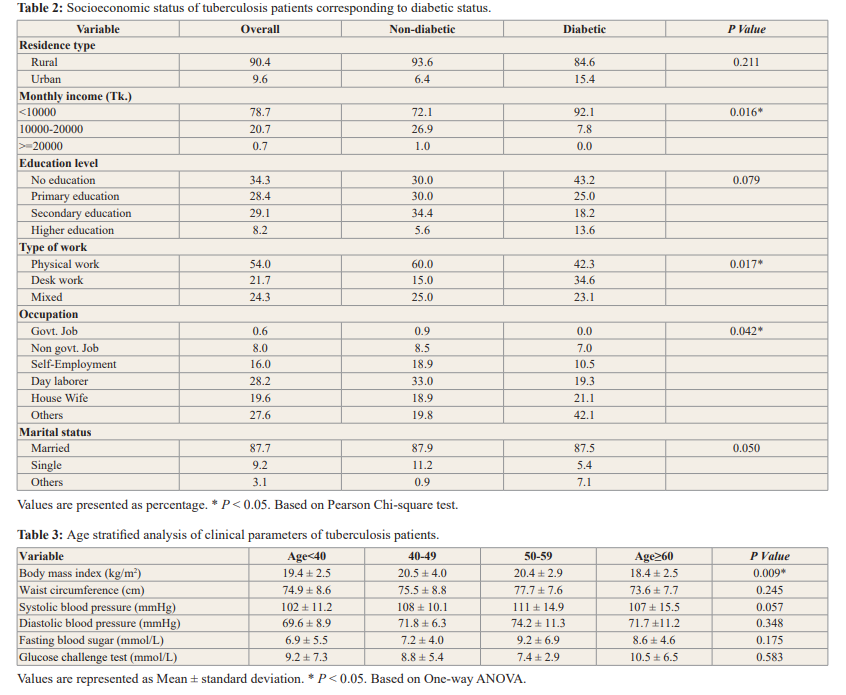
Table 2 demonstrates that more than 90% of the study participants are from rural areas. Approximately 80% of the research participants had a monthly income of less than BDT.10000/-. Illiteracy affects one-third of all cases. A considerable portion of the population works as day laborers. Socioeconomic status was also found to be significant as those who were earning <10000 Tk. per month were more prevalent to DM and TB as compared to those earning between 10000-20000 Tk. The various socio-demographic parameters that have been compared between respondents diabetic and non-diabetic groups. In the overall education level, 34% had no education, primary education 28.4%, secondary education 29.1%, and higher education was 8.2%. There was a significant difference between diabetic, and non-diabetic group for monthly income (p =0.016), type of work (p=0.017) and occupation (p=0.042). There was no significant difference between residence type (p=0.211), education level (p=0.079) and marital status (p=0.050) (Table-2).
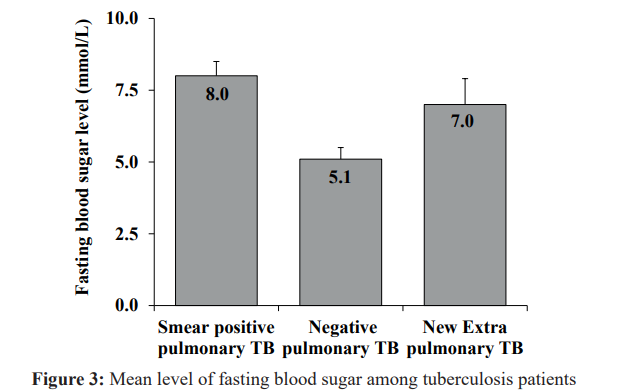
According to tuberculosis patients age group <40 had a lower level of prevalence of diabetes compared to the other groups.
Values are presented as mean ± standard deviation for continuous variables and percentage (%) for categorical variables. * P < 0.05. Based on T-test for continuous variable and Pearson Chi-square test for categorical variable.
There was statistical significance difference between the groups (Figure 2).
The clinical characteristics of subjects were explored when stratified into levels of tuberculosis patients. Tuberculosis patients was respondents into 4 groups by age. The only statistically significant result was in body mass index (p=0.009). There was no statistical difference between the subjects in age groups while talking about the waist circumference (p=0.245), systolic blood pressure (p=0.057), diastolic blood pressure (p=0.348), fasting blood sugar (p=0.175) and glucose challenge test (p=0.583) in tuberculosis patients (Table-3).
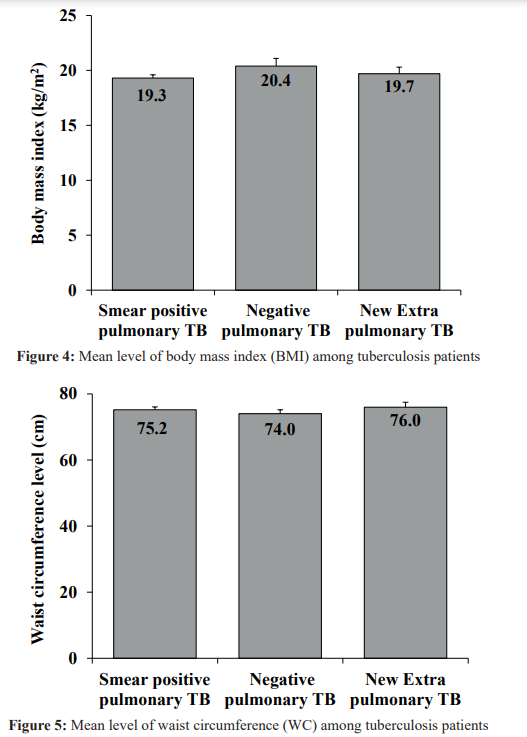
Mean level of fasting blood sugar are higher in smear positive pulmonary and lower-level negative pulmonary TB. (Figure 3).
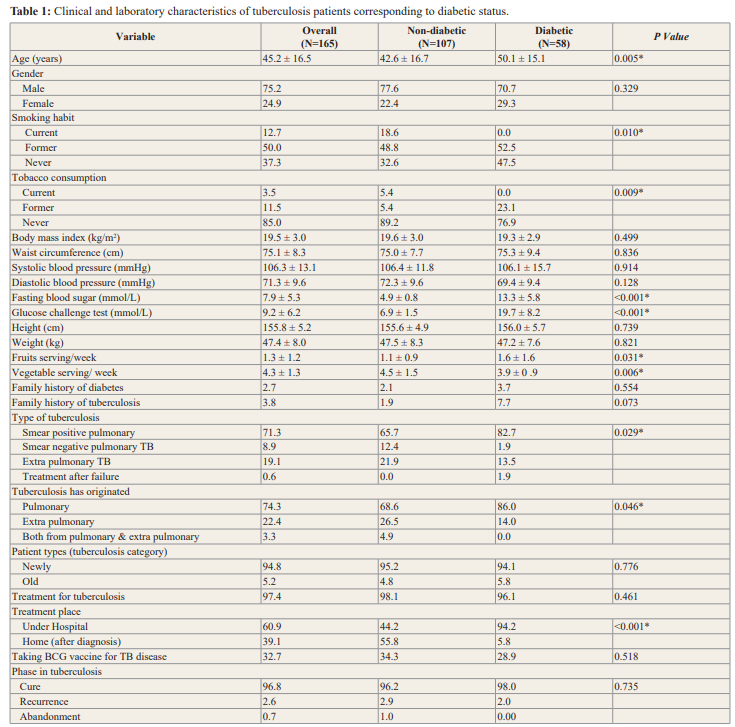
Mean level of body mass index (BMI) among tuberculosis patient smear positive pulmonary 19.3, negative pulmonary TB 20.4 and new extra pulmonary TB were 19.7 (Figure 4).
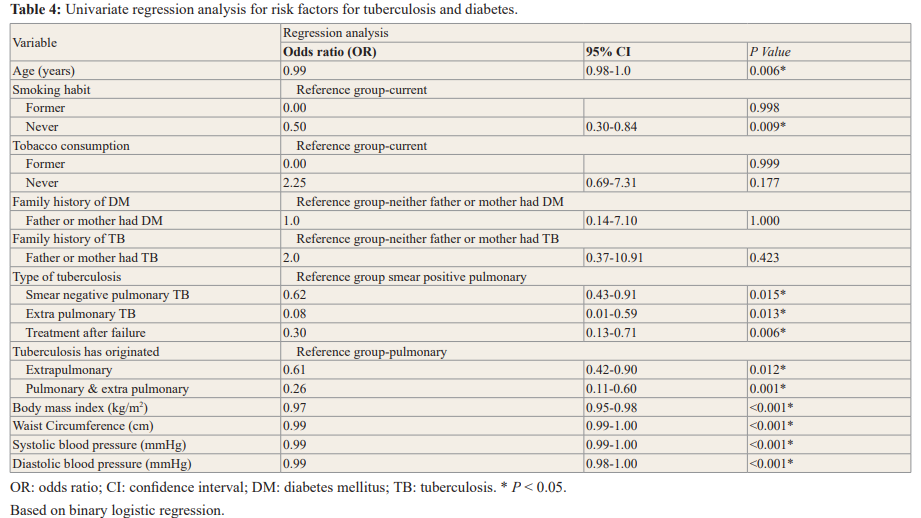
In the current study, smoking habit, tobacco consumption, family history of diabetes and family history of tuberculosis were not significantly linked with the presence of diabetes in tuberculosis patients; however, in binary logistic regression, age, type of tuberculosis, its origin (pulmonary or extra pulmonary), on the Pearson Chi-square test, remained significant.
Subjects in the type of tuberculosis smear negative pulmonary TB had an odds ratio (OR) of 0.62 (CI 0.43-0.91, p=0.015), extra pulmonary TB odds ratio (OR 0.08, CI 0.01-0.59, p=0.013) and treatment after failure odds ratio (OR 0.30, CI 0.13-0.71, p=0.006). Waist Circumference (OR 0.99, CI 0.99-1.00, p < 0.001) and body mass index (OR 0.97, CI 0.95-0.98, p <0.001) (Table 4).
Mean level of waist circumference (WC) among tuberculosis patient, smear positive pulmonary 75.2, negative pulmonary TB 74.0 and new extra pulmonary TB were 76.0 (Figure 5).
Discussion
In the current study, the DM prevalence in TB patients is found to be 35%. This finding is comparable with the study’s results conducted in India (29%) [16]. Diabetes was shown to be substantially more prevalent among elderly tuberculosis patients in this investigation. Several countries, including Indonesia, Saudi Arabia, and Taiwan, have found similar findings [17-19]. Globally, as life expectancy increases, health care availability improves, and the older population grows, the sheer number of diabetes patients will climb exponentially. Routine DM screening of tuberculosis patients will aid in the early detection of diabetes and pre-diabetes, allowing for the successful implementation of primary preventive strategies.
There was no significant connection between BMI or waist circumference and diabetes in this research. Several studies have found that people with tuberculosis and diabetes mellitus are considerably underweight and experience more weight loss [20,21].
This study evaluated the prevalence of diabetes mellitus in tuberculosis patients, but further research is needed to determine the optimal time for screening, a practical and cost-effective technique of screening, and any subsequent adjustments to conventional care guidelines for individuals with both illnesses. Numerous problems in fundamental, applied, and operational research are awaiting solutions. Nevertheless, this convergence of two epidemics should serve as a wake-up call to all physicians and researchers to prepare for the challenge of TB and diabetes collectively in the patients. Being forewarned and prepared increases the likelihood of lowering the combined burden of disease associated with diabetes and tuberculosis.
Conclusion
Diabetes is now a very common co-morbidity in patients with tuberculosis. Using fasting blood sugar measurements to screen individuals with tuberculosis will aid in the early diagnosis of diabetes. In addition, strategies are required to guarantee that individuals with both diseases can receive the best treatment possible.
Acknowledgments
This research has been supported by Kiban C Grant (Japanese Ministry of Education and Science) no-19K11543.
References
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 2006; 3: e442.
- Wild S, Roglic G, Green A., et all. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004; 27: 1047-1053.
- King H, Aubert RE, Herman WH, et al. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes care. 1998; 21: 1414-1431.
- IDF International Diabetes Federation Diabetes Atlas. 2014. Retrieved April. 4, 2014.
- Hall V, Thomsen R, Henriksen O, et al. Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic BMC public health. 2011; 11: 564.
- Chakaya J, Khan M, Ntoumi F, et al. Global Tuberculosis Report 2020–Reflections on the Global TB burden, treatment and prevention efforts. International Journal of Infectious Diseases. 2021; 113: S7-S12.
- Basnyat B, Caws M, Udwadia Z, et al. Tuberculosis in South Asia: a tide in the affairs of Multidisciplinary respiratory medicine. 2018; 13: 10.
- Noubiap JJ, Nansseu JR, Nyaga UF, et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2· 3 million patients with tuberculosis. The Lancet Global Health. 2019; 7: e448-e460.
- Shrestha N, Mishra SR, Ghimire S, et al. Burden of Diabetes and Prediabetes in Nepal: A Systematic Review and Meta- Analysis. Diabetes Therapy. 2020; 11: 1935-1946.
- Jayawardena R, Ranasinghe P, Byrne NM, et al. Prevalence and trends of the diabetes epidemic in South Asia: a systematic review and meta-analysis. BMC public 2012; 12: 380.
- Behera TB and diabetes--the dual epidemic: is it a matter of concern?. 2011; 58: 143-147.
- Workneh MH, Bjune GA, Yimer SA, et al. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara Region, Ethiopia: a cross sectional study. PloS one. 2016; 11: e0147621.
- Ogbera AO, Kapur A, Abdur-Razzaq, H, et Clinical profile of diabetes mellitus in tuberculosis. BMJ Open Diabetes Research and Care. 2015; 3: e000112.
- Hassmiller The association between smoking and tuberculosis. saludpública de méxico, 2006; 48: S201-S216.
- National Programme for Prevention and Control of Diabetes, Cardiovascular Disease and Stroke: A Manual for Medical Officer. Government of India – WHO Collaborative
- Vasudevan K, Govindarajan S, Chinnakali P, et Prevalence of diabetes mellitus among tuberculosis patients in Urban Puducherry. North American Journal of Medical Sciences. 2014; 6: 30-34.
- Singla R, Khan N, Al-Sharif N, et al. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2006; 10: 74-79.
- Alisjahbana B, Sahiratmadja E, Nelwan EJ, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clinical infectious diseases:an official publication of the Infectious Diseases Society of America. 2007; 45: 428-435.
- Chang JT, Dou HY, Yen CL, et al. Effect of type 2 diabetes mellitus on the clinical severity and treatment outcome in patients with pulmonary tuberculosis: a potential role in the emergence of multidrug-resistance. Journal of the Formosan Medical Association. Taiwan yizhi. 2011; 110: 372-381.
- Amrit G, Ashok S. Tuberculosis and diabetes: an appraisal. Indian Journal of Tuberculosis. 2000; 47: 3-8.
- Ponce-De-Leon A, Garcia-Garcia MdMde L, Garcia-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004; 27: 1584-1590.