Prevention of Diabetic Nephropathy by Low Protein Processed Genmai (Brown Rice)
Author(s): Shaw Watanabe1*, Keika Adachi2, Kunitolshi Iseki3
1Tokyo University of Agriculture, Medical Rice Association, Tokyo.
2Keio University, School of Medicine, Tokyo.
3Nakamura Clinic, Urazoe, Okinawa.
*Correspondence:
Shaw Watanabe, Tokyo University of Agriculture, Medical Rice Association, Tokyo.
Received: 30 Nov 2023 Accepted: 29 Dec 2023
Citation: Shaw Watanabe, Keika Adachi, Kunitolshi Iseki. Prevention of Diabetic Nephropathy by Low Protein Processed Genmai (Brown Rice). Diabetes Complications. 2024; 8(1); 1-5.
Keywords
According to the survey results at the end of 2021, the number of dialysis patients was 349,700, and in addition to complications of metabolic diseases such as diabetes and hypertension, the number of senescing kidneys for which the primary condition is not precise is increasing in Japan. According to the facility survey results, at the end of 2022, the number of dialysis patients in their 90s also increased [1]. Diabetic nephropathy accounts for 39.0% of all dialysis patients, so it is essential to prevent progression to end- stage kidney disease.
The typical clinical course of diabetic nephropathy starts from trace albuminuria, followed by an annual increase in albumin excretion of 10-20% if untreated, and progression to manifest nephropathy with overt proteinuria after 10-15 years in type 1 diabetes mellitus [2]. When the disease progresses to overt nephropathy, eGFR declines by 2 to 20 mL/min per year, and more than half of the patients develop end-stage renal failure within ten years. On the other hand, in type 2 diabetes, the onset of diabetes is unclear, and albuminuria and proteinuria may already be present at the time of diabetes diagnosis. Still, once nephropathy develops, the clinical course is considered to be similar to that of type 1 diabetes. Observational studies of diabetic nephropathy have also shown that changes in albuminuria may be associated with renal outcome, and reducing proteinuria may be very effective in preventing CKD as a complication.
In the Japan Diabetes Complications Study on 1,558 patients with type 2 diabetes over about eight years, the frequency of albuminuria of 300 mg/gCr or more was 0.23% per year in the group with less than 30 mg/gCr. In contrast, it increased to 1.85% annually in the 30 to 150 mg/gCr group. The risk of having albuminuria level of 300 mg/gCr or higher in the 30-150 mg/gCr group was 8.45 times greater than that in the 30 mg/gCr or lower group [3].
Recent studies have shown that the remission rate from trace albuminuria to normal albuminuria ranges from 21% to 64% and is more frequent than the rate of progression to overt albuminuria [4]. Still, a lead time bias would be present. In a study of 216 patients with type 2 diabetes in Japan, remission of microalbuminuria was associated with glycemic, blood pressure, and lipid control and contributed to a better prognosis for renal function decline and CVD [5,6]. More recent studies have also shown remission of overt albuminuria by the therapy [7,8].
Multidisciplinary treatment of diabetic nephropathy in the guidelines are:
- Improvement of lifestyle
- Correction of hyperglycemia
- Correction of glomerular hypertension.
- Control of serum lipids
- Protein-restricted diet (0.8 g/kg/day).
The low-protein diet was the only effective treatment for kidney disease until the 1960s, but it is said to be ineffective for diabetic nephropathy. A meta-analysis by Zhu et al. [9] showed that diet did not affect type I and type II diabetic CKD. Still, 0.8 g/kg/day protein restriction is ineffective [10]. Cochran Review recommends 0.6 g/ kg/day in the consensus report [11]. The remission rate increased with the achievement of glycemic and blood pressure control goals, and a 50% or more significant reduction in urinary albumin content suppressed the rate of subsequent renal function decline [12]. In a study of 211 patients with type 2 diabetes in Japan, the remission rate of overt albuminuria was 58.3% after an average of 4.5 years of observation.
So, suppressing urinary albumin is essential to keep eGFR, and the overt nephropathy phase is a target period of dietary therapy (Figure 1).
A meta-analysis of randomized controlled trials of various therapeutic interventions in CKD showed that a reduction in albuminuria with pharmacotherapy was associated with a renal protective effect, and this association did not differ by the proportion of patients with diabetic nephropathy.
Pathophysiology of CKD
The condition of the glomeruli and renal tubules depends on different pathophysiology. Blood pressure is considerably related to the amount of glomerular filtration. When the blood pressure of the imported arterioles is high, it becomes a state of hyperfiltration. The eGFR can be 120 ml/min/1.73 m2 or more. Capillaries, endothelium, and basement membrane have negative charges under normal conditions, blocking the passage of albumin molecules. However, when excessive leakage occurs, the spacing between the foot processes opens, and proteinuria occurs [13]. In diabetes mellitus, glycating substances are deposited on the mesangial substrate, causing degeneration of the glomeruli and hyalinization. In a cohort study of type 2 diabetes, patients showed proteinuria ten years from the onset, increasing toward overt proteinuria in the next five years (Figure 1). According to the increase of proteinuria, eGFR dropped concomitantly. As the glomerular lesion progresses, the renal tubules are also affected due to the cessation of blood flow in the nephron.
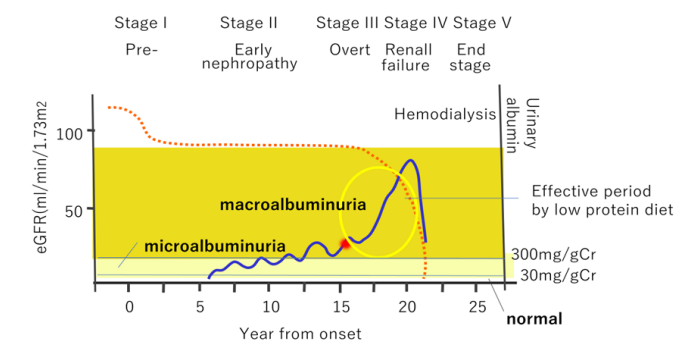
Figure 1: In case of diabetes mellitus, there is a medical history of 10–15 years before the appearance of overt proteinuria. Irreversible after overt nephropathy (especially when proteinuria is 1g/Cr or more or serum creatine is elevated) It is necessary to properly treat early nephropathy.
Nephrotoxin
Nephrotoxins should be a cause of CKD [14]. Indoxyl sulfate is a typical nephrotoxin, which comes from tryptophan metabolites indole by intestinal bacteria. It leaks into the liver and becomes indoxyl sulfate, further increasing nephrotoxicity. At that time, the liver also releases cytokines such as IL6 in response to inflammatory stimulation, posing a risk of cardiotoxicity (Figure 2a).
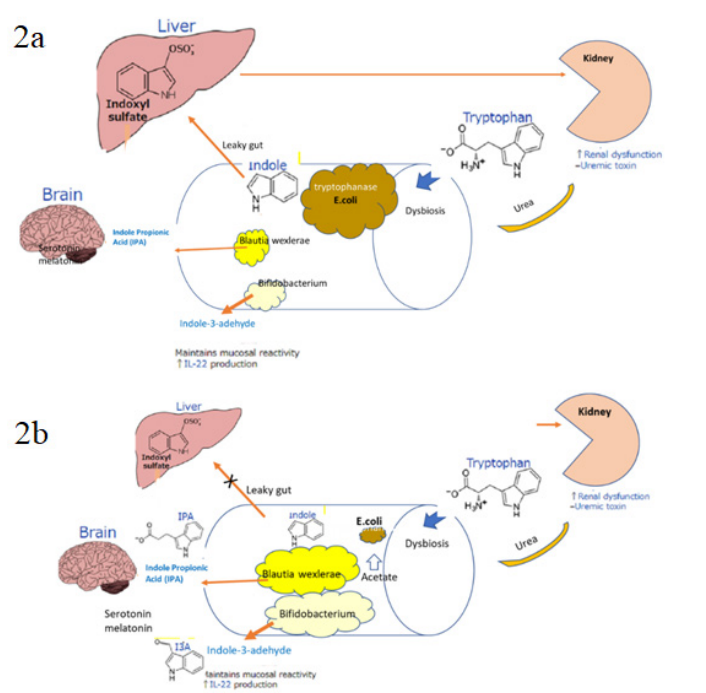
Figure 2a: In CKD, two pathological conditions, uremic dysbiosis and leaky gut progress CKD and cardiovascular accidents. Dysbiosis is associated with endotoxemia and chronic inflammation, disrupting the intestinal barrier and depletion of beneficial bacteria producing SCFAs. Drug therapy cannot stop the increase of end-stage renal disease. To quit the underlying negative spiral of a gut-kidney axis is essential.
2b: If brown rice can reduce protein, remaining dietary fiber and other functional ingredients could improve dysbiosis and leaky gut. High acetate and propionate lower pH of colon [15]. By correcting intestinal dysbiosis, tryptophan metabolism will be directed in a favorable direction, and renal dysbiosis will be improved. It also reduces the risk of cardiovascular disease by preserving and reducing inflammatory cytokines.
In 2021, an international panel of experts organized by Vanholder updated the definition of uremic toxins, subdividing medium molecules into small, medium-sized, and large medium molecules as a new classification and adding a new class of solutes with a size larger than the pores of the glomeruli. We have to evaluate the toxic damage caused by these molecules based on experimental and clinical evidence of their toxicity. Clinical assessments should focus primarily on relevant complex outcomes (mortality, cardiovascular and infectious complications, progression of renal function, cognitive decline). Still, patient-reported outcome measures (PROMs) concerning quality of life are essential.
It is necessary to reduce the nephrotoxin generated in the intestinal tract due to uremia, and several methods are employed. (1) Adsorption charcoal powder (Cremezin) has been developed to remove this nephrotoxin in the intestinal tract, but it isn't easy to swallow and has little effect [16,17]. (2) There was no significant difference in the results of clinical trials for hemodialysis to remove uremic toxins in the blood. Removal by the dialysis option is controversial. Because the metabolic processes that produce uremic toxins also have beneficial or essential compounds for the body's functioning. Substances made by tryptophan metabolism can regulate insulin sensitivity and serotonin precursors, such as indole-propionic acid and indole carbaldehyde, as well as toxins, such as indoxyl sulfate and kynurenine. Dialysis strategies produce positive and negative effects because they remove harmful and beneficial substances equally, without distinguishing between them, thus offsetting most of the benefits of toxin removal. (3) The administration of probiotics or prebiotics was tested to solve dysbiosis in some humans and animals and showed some efficacy. Still, since they are influential only during administration, they are not a fundamental method of preserving renal function in the long term [18].
The Negative Spiral of Entero-Renal Linkage That Progresses CKD
It is essential to cut off the negative spiral of the entero-renal linkage. Low-protein fermented brown rice (JAS0027) corrects dysbiosis and leaky gut and is the only food effective in preserving kidney function [19]. It is easy to incorporate into the patient's diet because it is delicious and easy to eat. Patients can reduce their protein intake by 10g daily by replacing their staple food with packed low-protein genmai rice. The diet needs to support the patient's self-directed efforts. Recommendations from an international workshop focused on diet are accepted widely [20].
Low-protein fermented brown rice JAS0027
Brown rice seems to be the most suitable for correcting the intestinal environment [21,22]. Therefore, the Medical Rice Association formed a consortium to conduct a study to remove protein from brown rice and reduce the protein content to less than one-tenth. The low-protein fermented brown rice (Gogyo-genmai) was produced by various technologies, from selecting raw rice, surface treatment with high-pressure steam, protein removal with lactic acid bacteria and enzyme solution, and hygienic rice cooking and packing [23]. In addition to the advantages of low- protein white rice, such as (1) energy similar to white rice, (2) protein content of less than one-tenth, (3) almost zero potassium, and (4) phosphorus less than one-quarter, this packed rice has the advantages of brown rice in that it has (5) dietary fiber, (6) ɤ-oryzanol, and (7) antioxidant capacity.
Low-protein fermented brown rice, which improves the intestinal microbiota, can relieve constipation, positively affects short- chain fatty acids by improving the intestinal microbiota, and can be expected to be effective in preserving kidney function (Figure 2b). Using this for three staple meals patients can reduce their protein intake by 10 g and have more room on the side meal menu. When they have a regular meal with their family, they only need to change the staple food with the same side dishes. It is easy for older people living alone at home to incorporate into their diet.
The diet of patients with CKD makes it challenging to control energy source intake and protein restriction at the same time. In a clinical trial at Keio University Hospital, a three-month intervention study with low-protein brown rice, in which patients were instructed to replace their staple food with low-protein brown rice packages without restricting side dishes, were divided into ten people who reduced their protein intake by 10 g or more as calculated, those who did not change much, and those who increased their protein intake. (Adachi et al., unpublished). In the group with reduced protein intake, constipation improved, E. coli and Akkermancia decreased due to an increase in acetic acid and propionic acid, and an increase in Blautia wexlerae, Blautia luti, and Bifidobacteria was observed. Acetic acid and butyrate in feces increased. Dysbiosis and leaky gut improved the tryptophan metabolic pathway, significantly reduced uremic toxins, and lowered markers of tubular disorders (Figure 3).
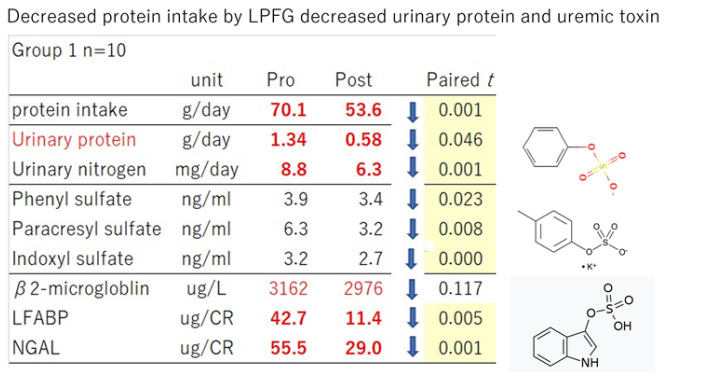
Figure 3: When we focus on Group 1, ten gram decreased protein intake by LPFG showed remarkable effects on the reduction of urinary protein, uremic toxins and biomarkers of tubular damage.
Suppose a 60-kg man who consumed 48 g of protein (0.8 g/kg body weight) reduced to 38 g (0.62 g/kg body weight) after a reduction of 10 g of protein intake. A 50 kg woman who consumes 40 g of protein loses 10 g, and her input becomes 30 g (0.6 g/kg body weight). The patient can achieve it more easily than with a diet that stoically targets 0.6 g. There are many randomized controlled trials of low-protein diets. Still, at the end of the study, the planned protein intake is usually higher, and the differences between the control groups are minor. By reducing their diet, others tend to have a lack of total energy intake, resulting in a state of malnutrition [24].
Therefore, most randomized controlled trials have failed since it is difficult to maintain the source of protein and program quantities of energy over the entire study period [25]. The results of randomized controlled trials involving many subjects could be more consistent. Individual differences are significant in the body's intestine-renal linkage and other metabolic networks, and subjects cannot be homogeneous. Preferences and acceptance of dietary advice are also influenced by personality, so doing a diet regimen that lasts several months with an RCT is a figment of the imagination. Comparing before and after interventions regarding cost and patient burden is a more direct and practical approach [26]. For that, the concept of medical rice among medical food may be necessary for future personalized dietary therapy because it works on prevention and treatment beyond the vague population- based concept of "risk reduction."
Cost of Hemodialysis
If CKD progresses and hemodialysis treatment is required, it will incur medical expenses of more than 5 million yen per person per year. Furthermore, if complications occur, it will not be enough to deal with medical costs alone and nursing care will be required. The person's and family member's quality of life will be significantly impaired. If patients are on hemodialysis, they will be issued a receipt for treatment for specified diseases. The government will pay in a particular case for long-term high-cost conditions under medical insurance, and the patients can reduce out-of-pocket payments to a maximum of 10,000 yen. In addition, self-reliance support medical care (rehabilitation and nurturing medicine) is subsidized by the national system for medical care received to reduce the "physical disability" of people with disabilities.
Diabetic patients who require dialysis treatment are defined as "physical disability level 1", so if they are issued a physical disability certificate, they will be eligible for subsidies for self- reliance support medical care. Those who have a disability certificate for grades 1 and 2 receive an expense subsidy system for severe physical and mental disabilities (a system subsidized by each prefecture or municipality), and many people have virtually no personal burden.
International Workshop on Dietary Therapy for CKD
The Ministry of Agriculture, Forestry and Fisheries (MAFF) has approved the Japan Agricultural and Forestry Standards (JAS0027) for low-protein processing and rice cooking methods. The International Workshop on Dietary Therapy was commissioned by the Medical Rice Association using the PRISM "Public- Private R&D Investment Expansion Program" last March [20]. The Medical Rice Association commissioned it to promote the international standardization of Japan's proprietary manufacturing technology, promote its dissemination, and contribute to expanding demand overseas, with the support of the Japanese Society of Anti- Aging Medicine and Japan Renal Disease Association. The second workshop shall be held in Okinawa on March 16-17, 2024, with Kunitoshi Iseki as the Chairman. Participants shall discuss more patient-friendly dietary therapy based on the clinical trials.
Conclusion
For chronic kidney disease (CKD), it is essential to correcting the entero-renal linkage! For diabetic patients, the overt proteinuria phase would be most effective for preventing progression to end- stage renal disease.
Intervention trials inferred that low-protein fermented brown rice is helpful for correct entero-renal linkage, and this standard is approved by the Japanese government (JAS0027). By replacing the three staple meals with this low-protein brown rice package, the patient can reduce protein intake by 10g. If the protein mass of a side dish per serving is about 10g, establishing a low-protein diet of about 0.6 g/kg body weight is easy. A voluntary low-protein diet with a low burden improves patients' quality of life and reduces medical costs. In the treatment of patients with diabetes and kidney disease, which is increasing around the world, it is necessary first to improve the patient's diet, which will not only reduce the burden on the patient himself but also improve his QOL, which will eventually lead to a reduction in medical costs.
References
- Hanafusa N, Abe M, Tsuneki N, et Current status of chronic dialysis therapy in Japan. J Society Dialysis. 2022; 55: 665- 723.
- de Boer IH, Afkarian M, Rue TC, et al. Diabetes Control and Complications Trial epidemiology of Diabetes Interventions and Complications Research Group Renal outcomes in patients with type 1 diabetes and J Am Soc Nephrol. 2014; 25: 2342-2350.
- Katayama S, Moriya T, Tanaka S, et al. Japan Diabetes Complications Study Group Low transition rate from normo and low microalbuminuria to proteinuria in Japanese type 2 diabetic individuals the Japan Diabetes Complications Diabetologia. 2011; 54: 1025-1031.
- Yokoyama H, Sone H, Oishi M, et Japan Diabetes Clinical Data Management Study Group Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes the Japan Diabetes Clinical Data Management study. Nephrol Dial Transplant. 2009; 24: 1212-1219.
- Araki S, Haneda M, Sugimoto T, et Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. 2005; 54: 2983-2987.
- Araki S, Haneda M, Koya D, et al. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 Diabetes. 2007; 56: 1727-1730.
- Yokoyama H, Araki S, Honjo J, et al. Association between remission of macroalbuminuria and preservation of renal function in patients with type 2 diabetes with overt Diabetes Care. 2013; 36: 3227-3233.
- Furuichi K, Shimizu M, Toyama T, et al. Research Group of Diabetic Nephropathy Ministry of Health Labour and Welfare of Japan Japan Diabetic Nephropathy Cohort Study study design methods and Clin Exp Nephrol. 2013; 17: 819-826.
- Zhu HG, Jiang ZS, Gong PY, et Efficacy of low protein diet for diabetic nephropathy a systematic review of randomized controlled trials. Lipids in Health and Disease. 2018; 17: 141- 150.
- Ideura T, Shimazu M, Morita H, et al. Protein intake of more than 0.5 g/kg BW/Day is not effective in suppressing the progression of chronic renal failure. Contributions Nephrology. 2007; 155: 40-49.
- Harn D, Hodson EM, Fouque D, et al. Low protein diets for nondiabetic adults with chronic kidney disease. Cochrane Database Syst Rev. 2018; 10: CD001892.
- Heerspink HJ, Kröpelin TF, Hoekman J, et al. Reducing Albuminuria as Surrogate Endpoint Consortium Drug induced reduction in albuminuria is associated with subsequent renoprotection a meta analysis. J Am Soc Nephrol. 2015; 26: 2055-2064.
- Miki The Essence of Filtration Barrier in the Podocyte Glomeruli. Clinical Functional Nutriology. 2009; 1: 226-227
- Vanholder R, Nigam SK, Burtey S, et al. What if not all metabolites from the uremic toxin generating pathways are toxic A hypothesis. Toxins. 2022; 14: 221.
- Li L, Ma L, Fu P, et al. Gut microbiota derived short chain fatty acids and kidney diseases. Drug Des Devel Ther. 2017; 11: 3531-3542.
- Akazawa T, Asano Y, Morita S, et al. CAP KD study group Effect of a carbonaceous oral adsorbent on the progression of CKD a multicenter randomized control trial. Am J Kidney Dis. 2009; 5: 459-467.
- Marier JF, lLee J, Kambhampati SRP, et Effect of repeated oral administrations of the oral adsorbent AST 120 on serum creatinine and other markers of renal function A randomized controlled study in patients with chronic kidney disease. Am J Nephrol. 2006; 26: 136-141.
- Mishima E, Fukuda S, Abe T, et al. Intestinal bacteria and chronic kidney disease Anti Aging Medicine 2016; 12: 18-23.
- Watanabe S, Adachi K, Wakino S, et A dietary therapy with low protein genmai to improve the gut kidney axis and reduce CKD progression. Asia Pac J Clin Nutr. 2022; 31: 341-347.
- Watanabe S, Kashiwara The International Conference on dietary therapy for chronic kidney disease with special reference to the gut kidney linkage and low protein brown rice. Diabetes Complication. 2023; 7: 1-3.
- Hirakawa A, Aoe S, Watanabe S, et The nested study on the intestinal microbiota in GENKI study with special reference to the effect of brown rice eating. J Obes Chronic Dis. 2019; 3: 1-13.
- Kikuchi K, Watanabe S, Matsuo M, et al. Changes in microbiota and short chain fatty acids following 3 month pilot intervention study feeding brown rice balls to healthy volunteers. Prensa Med Argent. 2021; 107: 315.
- Watanabe S, Minakuchi S, Yamaguchi M, et al. A new low protein foodstuff from processed brown rice for chronic kidney disease. Acta Scientific Nutritional Health. 2021; 5: 1-10.
- Levy AS, Greene T, Beck GJ, et Dietary protein restriction and the progression of chronic renal disease What have all MDRD study results shown. J Am Soc Nephrol. 1999; 10: 2426-2439.
- Watanabe S. A trap of RCT in low protein dietary therapy Reevaluation of pre post study for dietary intervention. Acta Scientific Nutritional Health. 2023; 7: 35-38.
- Ebell Evidence based practice outcome. College of Public Health University of Georgia Retrieved. 2011.