Probiotic Profiling of Selected Yogurt Drinks in Benin City, Edo-State, Nigeria
Author'(s): Ndeokwelu D1, Olawale O2, Alabi B3, Oluwatosin O3 and Osho P3*
1 Department of Microbiology, Benson Idahosa University, Edo State, Nigeria.
2 Department of Microbiology, University of Ibadan, Oyo State, Nigeria.
3 Department of Hematology and Virology, University of Medical Sciences, Ondo State, Nigeria.
*Correspondence:
Dr. Patrick O. Osho, Department of Hematology and Virology, University of Medical Sciences, Ondo State, Nigeria.
Received: 10 Jun 2022; Accepted: 19 Jul 2022; Published: 25 Jul 2022
Citation: Ndeokwelu D, Olawale O, Alabi B, et al. Probiotic Profiling of Selected Yogurt Drinks in Benin City, Edo-State, Nigeria. Clin Immunol Res. 2022; 6(1): 1-9.
Abstract
Introduction: Yogurt is a potential source of probiotic organisms, probiotic are viable microorganism that exhibit beneficial effect on the health of human beings, improving the intestinal microbial balance. This project examined the potential of yogurt drinks sold in Benin City to serve as probiotics beyond offering nutritional benefits.
Methodology: Eleven samples of different brands yogurt (Cami yogurt, Goon super yogurt, Solmax, La Cerem, Yugo, Hollandia, Elite, Blessed, Fresh yo, Life Nice, Sargo yogurt) were purchased from major markets in Benin City. Samples were inoculated on de Mann Rogosa Sharpe (MRS) agar at 37?C they were presumptively identified as Lactobacillus and Lactococcus species. These were tested for their antimicrobial activity against some pathogens of public health importance (Escherichia coli, Staphylococcus aureus, Proteus mirabilis and Pseudomonas aeroginosa).
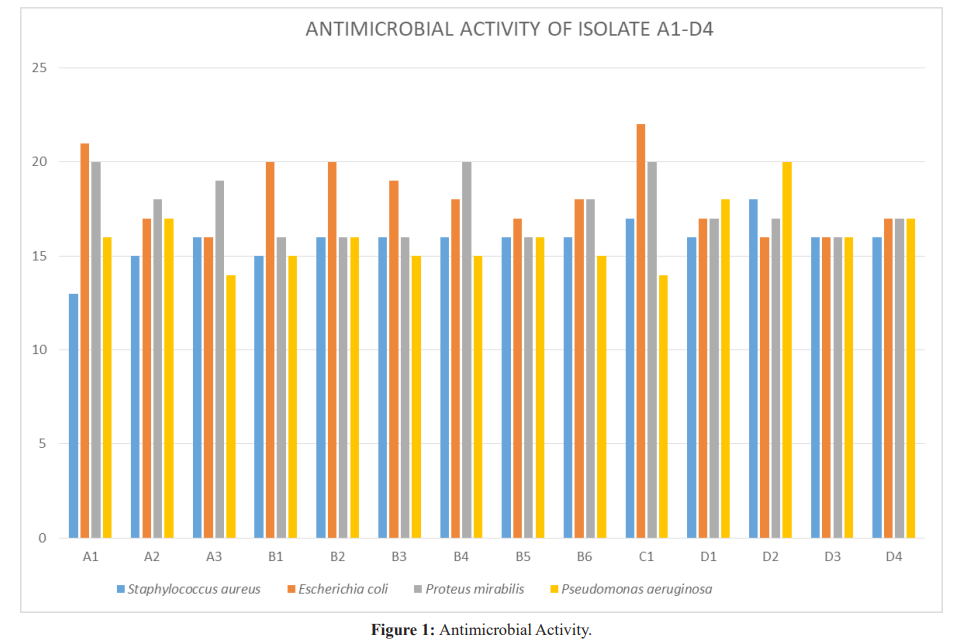
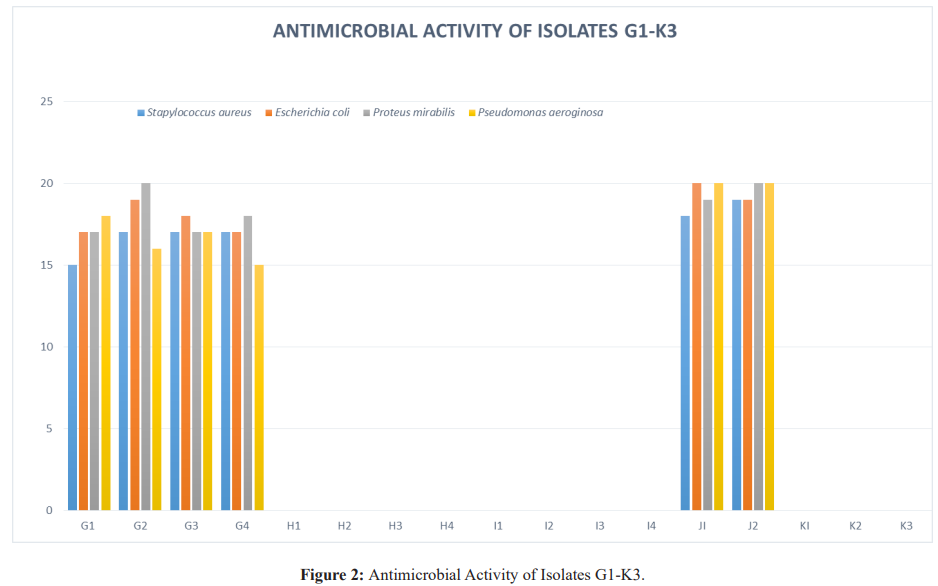
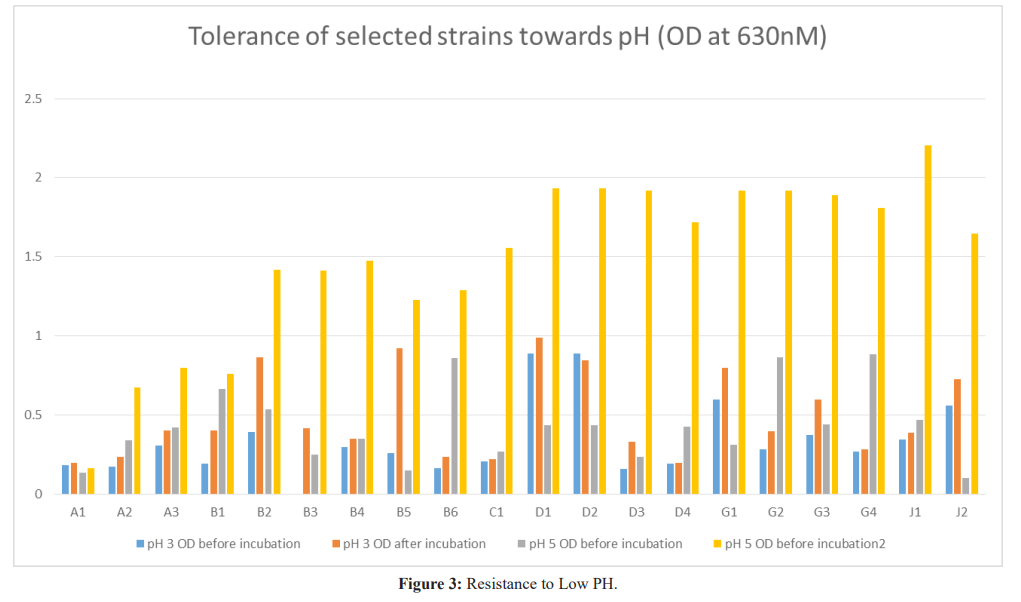
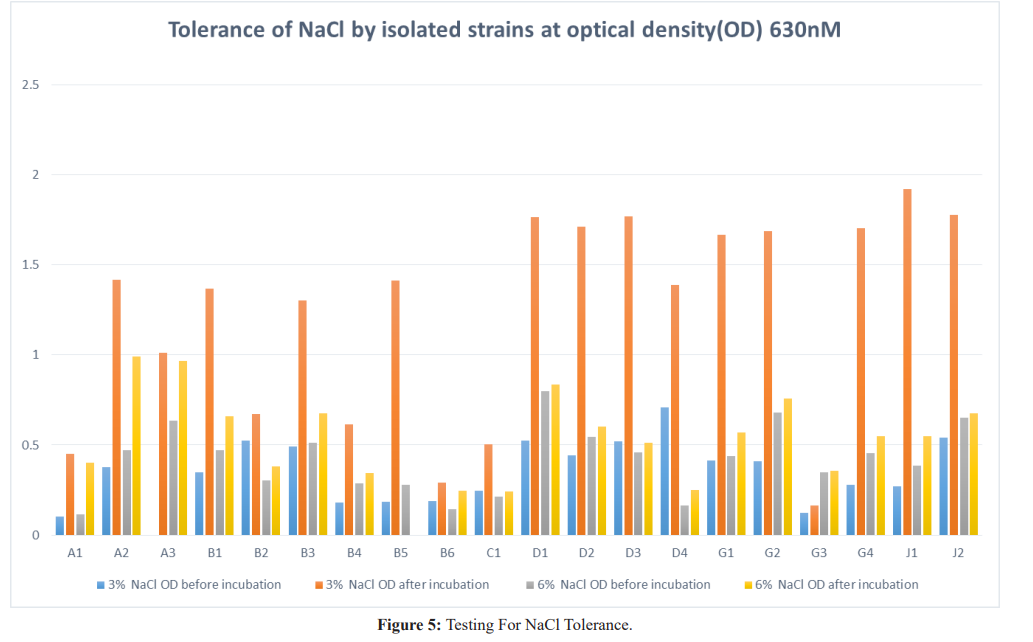
Results: Twenty isolates showed antimicrobial activity while eleven did not, for a proper probiotic; potential, inhibitory isolates were further tested for resistance to bile salt, sodium chloride and pH. Isolates J1 and J2 had the highest inhibitory activity, inhibiting all indicator organisms and they were from Life Nice yogurt while the least was D2 from Solmax yogurt. Isolates J1 and J2 grew best when inoculated into broth containing bile salt, NaCl and pH with A3 from Cami yogurt the lowest for bile salt, B6 from Goon super yogurt the lowest for NaCl and A1 the lowest for pH.
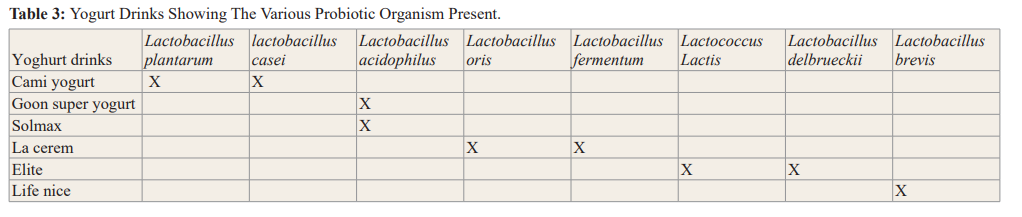
Conclusion: After the study was concluded out of the eleven yogurt drinks sampled only six (Life nice, Elite, La cerem, Solmax, Goon super and Cami yogurt) drinks could be classified as probiotics.
Keywords
Introduction
The term probiotic is defined as “a live microbial feed supplement which beneficially affects the host animal by improving its microbial balance’’ [1]. Probiotic bacteria may produce various compounds, which are inhibitory to growth of pathogens, which include organic acids (lactic and acetic acids) and bacteriocins. The organic acids not only lower the pH, thereby affecting the growth of the pathogen, but they can also be toxic to the microbes [2]. There is increasing evidence that probiotics are beneficial in gastrointestinal disturbances, such as diarrhea, dysentery, typhoid etc. [2].
The commercial utilization of probiotics isolated from traditional and naturally fermented dairy products, which possess health- promoting effects, which have proved to be beneficial to man. Research on probiotics isolated from such traditional and naturally fermented dairy products reveals a long history of safe use. Commonly claimed benefits of probiotics include the decrease of potentially pathogenic gastro-intestinal microorganisms, the reduction of gastro-intestinal discomfort, the strengthening of the immune system, the improvement of the skin's function, the improvement of bowel regularity, the strengthening of the resistance to cedar pollen allergens, the decrease in body pathogens, the reduction of flatulence and bloating, the protection of DNA, the protection of proteins and lipids from oxidative damage, and the maintaining of individual intestinal microbiota in subjects receiving antibiotic treatment. Scientific evidence to date has however been insufficient to substantiate any anti-disease claims or health benefits from consuming probiotics [3].
The most commonly known probiotic organisms are the Lactic acid bacteria (LAB) including Lactobacillus, leuconostoc, Lactococcus, pediococcus and Bifidobacterium ,Which are naturally found in some dairy products, decaying plants, intestinal tract and mucous membranes of animals and humans [4]. Examples of probiotic products include forever-active probiotic, healthy origin probiotic, trubiotics, align probiotic supplements. There are conditions (e.g. antibiotic stress, HIV/AIDS) when the load of LAB in the body system becomes very low or virtually absent that exogenous sources are required to improve the immune function and prevent infection.
Yogurt is one of the best-known foods that contain probiotics [5]. Yogurt is a non-alcoholic beverage that plays a very important role in the dietary pattern of Nigerians. Yogurt is a food produced by bacteria fermentation of milk. The bacteria used to make yoghurt are known as "yogurt cultures". Fermentation of lactose by these bacteria produces lactic acid, which acts on milk protein to give yogurt its texture and its characteristic tang. Worldwide, cow's milk, the protein of which is mainly casein, is most commonly used to make yogurt. Milk from water buffalo, goats, ewes, mares, camels, and yaks however, is also used to produce yogurt in various parts of the world [6].
In addition to the positive impact of yoghurts on pathogenic microorganisms, yoghurts are nutritionally rich in protein, calcium riboflavin, Vitamin B6 and vitamin B12. It has nutritional benefits beyond those of milk, lactose intolerant individuals can sometimes tolerate yogurt better than other dairy products because the lactose in the milk is converted to glucose and galactose, and partially fermented to lactic acid by the bacteria culture [7]. Probiotic yoghurts are known to reduce the total cholesterol level in the body while increasing the good cholesterol level in the body. They are also known to reduce the risk of type 2 diabetes [7].
Aim and Objectives
Yogurts drinks are the most common source of probiotics and many yoghurt brands are available in Benin City proffering nutritional value to consumers.
Therefore, this project was aimed at screening and examining yogurt drinks sold in Benin City for their potential to serve as probiotic drinks beyond offering only nutritional benefit.
The specific objectives include the following
- To screen the yogurt drinks sold in Benin City for presence of beneficial organisms
- To identify the probiotic microorganisms found in the yogurt
- To screen for antimicrobial activity of the
- To screen for bile salt tolerance for isolated
- To screening for acid tolerance of isolated
- To screening for sodium chloride (NaCl) tolerance of
Materials and Method
Collection of Samples
Eleven brands of commercially produced yogurt drinks (Cami yogurt, Goon super yogurt, Solmax, La Cerem, Yugo, Hollandia, Elite, Blessed, Fresh yo, Life Nice, Sargo yogurt) were purchased randomly at major markets in Benin City, Edo state and immediately transported to the laboratory for microbial analysis.
Culture Media
Culture media used were de Mann Rogosa Sharpe (MRS) agar and broth and Muller Hilton agar. The media was prepared according to the manufacturer’s instructions. After homogenization in boiling water, the media were sterilized by autoclaving at 121Ë?c for 15 minutes at a pressure of 15 pounds/sq inch.
Isolation of Bacteria
A ten-fold serial dilution of samples was done by adding 1ml of the sample into 9ml of sterile distilled water. Further dilutions were made up to 10-3. 1ml of each dilution was inoculated into de Mann Rogosa Sharpe agar using pour plate method. The inoculated plate were incubated at 37Ë?C, under aerobic conditions. After 24 hours, the plates were examined for growth and single representative colonies were randomly selected and subcultured to obtain pure cultures for identification.
Identification of Isolates
Identification of isolates was carried out based on morphological, physiological and biochemical characteristics as described in Bergey’s manual of Determinative Bacteriology. The test employed were Gram reaction, endospore, motility, catalase, Indole, methyl red, voges proskauer, sugar fermentation tests.
Morphological Characterization
Cultural Characteristics
The colonial characteristics of the isolates on the culture plates were examined for their shape, size, elevation, pigment, surface, margins and texture.
Gram Staining
A smear of the isolate was made on a clean glass slide, heat fixed and flooded with crystal violet for 30 seconds. It was rinsed with water, covered with iodine for one minute, rinsed again and then decolorized with 95% ethanol for 15-30 seconds. The ethanol was rinsed off and the slide was flooded with safranin for 60-80 seconds. This was followed by a final rinsing after which the slides were allowed to air dry and then viewed under the microscope. The colour, shape and arrangement of the cells were noted.
Motility
This was done by the stab culture technique. The isolates were inoculated into a semi solid medium in test tubes by making a straight line stab up to about the center of the medium and were incubated at 37Ë?c. After 18-24 hours of incubation, the tubes were examined for growth. Growth along the line of stabbing indicates negative result, while concentration of growth at the top of the tube or turbidity of the whole medium indicates positive result for motility.
Biochemical Characterization
Catalase Test
This was used to determine the production of catalase by the isolate. A few drops of 3% hydrogen peroxide was placed on a clean glass slide and then a loopful of the isolate was smeared onto the hydrogen peroxide on the slide. Positive result was indicated by effervescence.
Indole Test
This was used to determine the ability of each isolate to produce indole from the amino acid tryptophan. Peptone water (1%) was prepared and distributed into test tubes and sterilized. After cooling, the tubes were inoculated and incubated at 37Ë?C for 48 hours. Kovac’s reagent (0.5mL) was added and gently shaken and the tubes were observed for the appearance of a red colour, which indicates positive result, while negative result is indicated by absence of a red colour.
Methyl-Red and Voges Proskauer Test
This is a two in one test and was used to determine the kind of products of fermentation of glucose by the isolates, such as highly acidic compounds like lactic, acetic and formic acids or acidic compounds like butandiol and acetion. The isolates were inoculated in test tubes of MR-VP medium and incubated at 37Ë?c for 48 hours after which one third of each culture was aseptically transferred into other sterile test tubes. To the first test tube, 5 drops of methyl red was added and was examined for a red colouration, which indicates positive methyl red test. To the other one-third 0.6mL of Barrit’s reagent a (6% α-naptol) and 0.2mL of Barrit’s reagent B (potassium hydroxide) was added and observed for red colouration, immediately and within 20 Minutes, which indicates positive Voges proskauer test.
Endospore Staining
A smear of the isolate was made on a slide, heat fixed and then placed on a boiling water bath. Malachite green was added to the slide continuously for ten minutes without allowing it to dry off after which it was counterstained with safranine for 15 seconds. The slide was allowed to air dry and was observed for presence and position of endospore under the microscope. The endospore takes up the malachite green while the cells takes up the safranine. Hence, the presence of green spot within the red cell indicate positive result, while absence of green spot indicates negative.
Ammonia Production from Arginine
Heat to melt the agar and dispense 4mL per 100mm disposal tube, autoclave the medium. Stab inoculated tube in duplicate then seal one tube with about 1cm depth of sterile mineral oil. Incubate for 3-7days at 37Ë?c. The enzyme arginine dihydrolase releases ammonium from arginine. The resulting alkalinity is indicated by the pH indicator bromo-cresol purple. Which turns dark pink colour under oil as opposed to the purple colour of un-inoculated controls. The unsealed inoculated tube will turn pink but is only to serve as a positive control.
Sugar Fermentation Tests
This was used to determine the ability of the isolates to ferment carbohydrate, producing either acid or acid and gas. The isolates were grown on peptone water containing only the sugar to be tested as carbon source. Bromo-cresol purple was added as indicator of pH change and the production of the gas detected by inserted inverted Durham tubes. After 24-28hours of incubation at 37Ë?C, the broth cultures were observed for colour change from purple to yellow and trapping of gas in Durham tubes, which indicates positive result and no colour change indicates negative result. The sugars used were glucose, fructose, lactose, sucrose, galactose, xylose and mannitol.
Confirmatory Test for Obtained Indicator Organisms
Some bacterial species used as indicator organisms during the antimicrobial test were obtained for Faith Mediplex laboratory, Benin City. Some confirmatory tests such as gram staining, coagulase production, growth in differential media, Indole production, Voges-Proskauer and sugar fermentations test were carried out on the organisms.
Probiotic Properties of Isolates
For the determination of probiotic properties of isolates these major selection criteria were chosen. Antimicrobial activity, growth in different concentration of sodium chloride, resistance to low pH, tolerance against bile salt and the antimicrobial activity agar well diffusion method was used to detect the antimicrobial activity of all the isolated strains, against indicators of public health importance pathogens such as Escherichia coli, Staphylococcus aureus, Proteus mirabilis and Pseudomonas aeroginosa. Briefly, 0.1ml of different pathogens was spread on Mueller Hinton agar plates in which wells with a diameter of 8mm were made and filled with 100µl of live suspension of probiotic culture. Plates were incubated at 37ºC for 24 h and the zone of inhibition was recorded. The strain which showed potent antagonistic activity against indicator pathogens were assessed further for their probiotic potential [8].
Growth at Different Concentration of Sodium Chloride (NaCL) The isolates were tested for growth at different concentration of sodium chloride. In this 1mL of overnight culture of the isolate were inoculated into 5mL of MRS broth containing 3% and 6% of NaCl. The broths were incubated at 37oC and was monitored by change in density using a Lambda 25 UV/visible spectrophotometer at 630nM [8].
Testing for Acid Tolerance
The effect of low pH on the survival of lactobacilli was examined by inoculating 1% of freshly prepared seed culture in 9mL of MRS broth with pH of 2.0 and 5.0 followed by incubation at 37oC for 24 hours. Growth was measured by taking the optical density before and after incubation for 24hours. Increase in the optical density was considered as a reflection of growth at 37Ë?C [8].
Testing for Bile Tolerance
The effect of bile on the survival of isolates was examined by inoculating 1mL of 24hours culture into MRS broth containing oxbile (2%w/v) followed by incubation at 37Ë?c. Growth was measured by taking the optical density before and after incubation for 24hours. Increase in the optical density was considered as a reflection of growth at 37oC [8].
Results
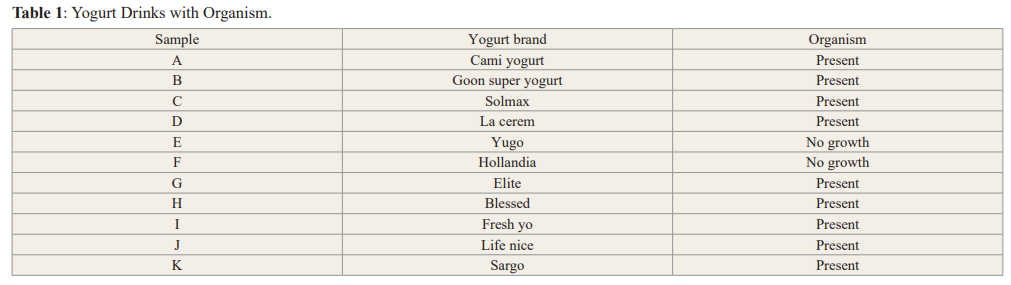
Nine of the brands of yogurt contained live organisms, which could be referred to as their starter cultures while two contained none. Thirty one (31) isolates were screened from the nine yogurt samples but only those with antimicrobial activity were identified. Twenty (20) lactic acid bacteria (rod and Cocci shaped) were isolated from different yogurt samples marketed in Benin City.
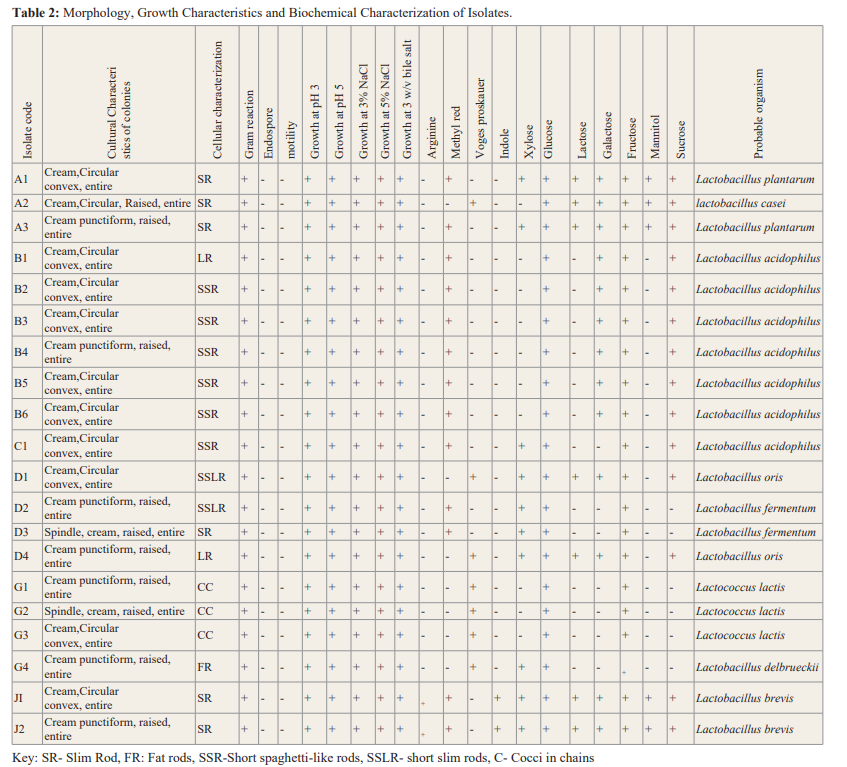
The results of morphology of all the isolates showed small, medium and large colonies in size, creamy in colour, entire margins and flat, convex colonies. Gram staining showed gram-positive rods and cocci of different lengths, some thin, fat and in pairs and clusters, while others were slender shaped.
The biochemical tests carried out revealed that all the isolate were indole, motility, endospore and catalase negative. They varied in the results for methyl red, voges proskauer and arginine. The isolates also showed different patterns to sugar fermentation, as they all fermented glucose and fructose while they varied in the fermentations of sucrose, galactose, xylose, sucrose and mannitol.
Discussion
In this study, the probiotic properties of the yogurt drinks sold in Benin City were examined. The first criteria was to check for the presence of beneficial microorganisms in the eleven (11) yogurt drinks sampled. The result showed the occurrence of two lactic acid bacteria (LAB) Lactobacillus sp (rod shaped) and Lactococcus sp (cocci-shaped). This is similar to findings by Ashamar, [9] that these bacteria are commonly found in yogurt drinks. However, Lactobacillus were dominant with a ratio of 8:1.
Antimicrobial activity is one of the most important selection criteria for probiotics. In this study the antimicrobial activity of isolated strains were tested against indicator organisms such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeroginosa and Proteus mirabilis, similar organisms was used by Dunne et al. [10] and Chuayana et al. [11]. Gram positive and Gram-negative organisms were selected as indicator organism, to check for the spectrum of activity of the thirty one (31) isolates; only twenty (20) strains were able to inhibit the growth of the indicator organisms. This is similar to the report by Dunne et al. [10]. All the isolated strains showed broad spectrum of activity against the indicator organisms, similar findings were made that strains isolated from yogurt had broad spectrum of activity. The eleven isolates, which lacked antimicrobial action, were discarded and the twenty remaining isolates were further investigated for their more probiotic properties.
Being resistant to low pH is one of the major selection criteria for probiotic strains. Since, to reach the small intestine they have to pass through from the stressful conditions of the stomach. Although in the stomach, pH can be as low as 1.0, in most in vitro assays pH 3.0 has been preferred. This is because a significant decrease in the viability of strains is often observed at pH 2.0 and below. For selection of strains resistant to low pH, the pH of the broth was adjusted to 3.0 and 5.0. To show probiotic sufficiency, they should reach to the lower intestinal tract and maintain themselves over there. This is supported by Koll et al. [8]. The isolated strains all grew at both pH 3 and 5, although the increase in optical density recorded at pH 5 was higher than that of pH 3. Lactobacillus brevis had the highest growth rate at both pH and Lactobacillus fermentum had the lowest growth rate at both pH 3 and pH 5. This is supported that Lactobacillus species has high survival rate at low pH.
Strains need to be resistant to the stressful conditions of the upper intestine, which contain bile. To show probiotic sufficiency, they should reach the lower intestinal tract and maintain themselves
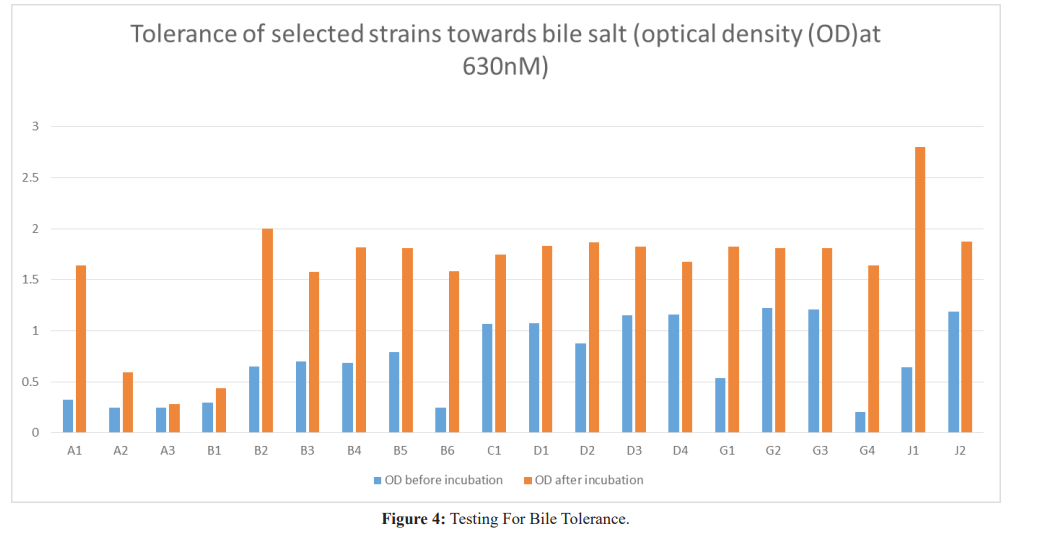
over there. For a study the strains were tested at 2% (w/v), which is the range used for most in vitro test. Of all the isolated strains, Lactobacillus brevis had the highest growth rate after 24 hours of incubation with Lactobacillus plantarum having the lowest growth rate. This is similar to findings by Chou and Weimer [12] that lactic acid bacteria isolated from various sources are able to grow in the presence of bile salt.
Tambekar [2] reported that the three isolated strains from yogurt showed excellent probiotic acid tolerance at pH 3.0 and bile salt tolerance at 2.0%. Tolerance to bile salts is a prerequisite for colonization and metabolic activity of bacteria in the small intestine of the host. This will help Lactic acid bacteria to reach the small intestine and colon and contribute in balancing the intestinal microflora [2].
NaCl is an inhibitory substance, which may inhibit growth of certain types of bacteria. In present study, the lactic acid bacteria isolates were able to tolerate 3% and 6% NaCl concentration. If the lactic acid bacteria were sensitive to NaCl then it would not be able to show its activity in the presence of NaCl so it was essential to test the NaCl tolerance of lactic acid bacteria isolates. Lactobacillus brevis had the highest growth rate at 3% concentration of sodium chloride. The present experimental results were similar to the work done by Adebayo-tayo and Onilude [13].
Conclusion
The bacteria isolates from yogurt were characterized based on their probiotic properties. All strains survival was accessed under conditions simulating the human gastrointestinal tract. They were all found to be resistance to low pH, bile salt, NaCl and they had inhibitory activity. It was concluded that the LAB species identified by this work could be used as probiotic. It was safely concluded that even if some yogurt drinks lack beneficial organism, other yogurt drinks contain probiotic organisms.
I therefore recommend that yogurt is a good source of probiotics that have health benefits and that these drinks when taken could limit the occurrence of diseases caused by pathogens such as the ones used in this study. Yogurt can therefore be said to be good source of probiotics and I advise yogurt producers who do not use starter cultures in production to begin adding because of their beneficial effects on humans.
Furthermore, of all the yogurt drinks, assessed life nice yogurt can be recommended as the yogurt carrying the highest probiotic properties and Cami yogurt had the least probiotic property.
References
- Aslam S, Qazi JI. Isolation of acidophilic lactic acid bacteria antagonistic to microbial contaminants. Pakistan Journal of Zoology. 2010; 42: 567-573.
- Tambekar DH, Bhutada SA. Studies on antimicrobial activity and characteristics of bacteriocins produced by Lactobacillus strains isolated from milk of domestic animals. The International journal for Microbiology. 2010; 8: 1-6.
- Savadago A, Traore SA. Bacteriocins and lactic acid bacteria – a minireview. African Journal of Biotechnology. 2006; 5: 678-683.
- Anderson DG, Nester EW, Pearsall NN, et al. Lactic acid bacteria. In: Microbiology; A Human Perspective, 2nd e.d. McGraw-Hill Companies. 1998; 567.
- Meydani SN, Oskar A, Russell RM. Yogurt and gut function. America Journal on Clinical Nutrition. 2004; 8: 245-256.
- Aouji M, Kolars JC, Levitt MD, et al. Yogurt an Autodigesting Source of Lactose. New England Journal of Medicine. 1984; 3: 1-3.
- Analie L, Bennie CV. Yogurt as probiotic carrier food. International Dairy Journal. 2001; 1: 1-17.
- Koll P, Mander R, Macotte H, et al. Screening & evaluation of human intestinal Lactobacilli for the development of novel gastrointestinal probiotics. Current Microbiology. 2010; 61:139-147.
- Asmahan AA. Isolation and identification of Lactic Acid Bacteria isolated from traditional drinking yoghurt in Khartoum state, Sudan. Current Research in Bacteriology. 2011; 4: 16-22.
- Dunne C, Murphy L, Flynn S, et al. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie van Leeuwenhoek. 1999; 76: 279-292.
- Chuayana JEL, Ponce V, Rivera C, et al. Antimicrobial activity of probiotics from milk products. Philadelphia Journal of Microbiology on Infectious Diseases. 2003; 32: 71-77.
- Chou LS, Weimer B. Isolation and characterization of acid and bile tolerant isolates from strains of Lactobacillus acidophilus. Journal of Dairy Science. 1999; 82: 23-31.
- Adebayo-Tayo BC, Onilude AA. Screening of lactic acid bacteria strains isolated from some Nigerian fermented foods for eps production. World Applied Sciences Journal. 2008; 4: 741-747.