Profiles of Autoantibodies and Markers of Inflammation in Patients with Systemic Lupus Erythematosus (SLE) in Abidjan
Author'(s): Goran-Kouacou Amah Patricia Victorine1 *, Dassé Séry Romuald1 , Yéboah Oppong Richard2 , Okoué Djédji1, Adou Adjoumanvoulé Honoré1 , Assi Aya Ursule Aniela1 , N’Guessan Koffi1 , Séri Yida Jocelyne1 , Koya Herbert Gautier2, Mémel Lasme Roselle Charline2, Moussa Salimata1, Oura Brou Doris1 and Siransy Kouabla Liliane1
1 Immunology and Allergology Department, Medical Sciences Training and Research Unit, at Félix Houphouët-Boigny University, located at the Boulevard de l’Université in Cocody, BPV34 Abidjan Côte d’Ivoire.
2Immunology and Allergology Department, Alassane Ouattara University, Bouaké, Côte d’Ivoire.
*Correspondence:
Goran-Kouacou Amah Patricia Victorine, Immunology and Allergology Department, Medical Sciences Training and Research Unit, at Félix Houphouët-Boigny University, located at the Boulevard de l’Université in Cocody, BPV34 Abidjan, Côted’Ivoire, Tel +225 0709345052.
Received: 01 Sep 2023; Accepted: 08 Oct 2023; Published: 15 Oct 2023
Citation: : Goran-Kouacou APV, Dassé SR, Yéboah OR, et al. Profiles of Autoantibodies and Markers of Inflammation in Patients with Systemic Lupus Erythematosus (SLE) in Abidjan. Clin Immunol Res. 2023; 7(1): 1-6.
Keywords
Introduction
Systemic lupus erythematosus (SLE) is the prototype of a chronic systemic autoimmune inflammatory disease characterised by the production of a wide range of autoantibodies (Ab), complement activation and deposition of immune complexes in tissues leading to multivisceral lesions [1,2]. These auto-Ab are dominated by antinuclear Ab (ANA). Due to their high sensitivity in systemic autoimmune diseases (AIDs) (95%), ANA are a good screening test. However, due to their low specificity (Sp 40-50%) [2,3], their presence is only a diagnostic orientation criterion. It is therefore essential to identify the antigenic targets of these ANA, which are the double-stranded DNA of the nuclei (native DNA) and the soluble nuclear antigens (ENA, for "extractable nuclear antigens") [2,3]. Native anti-DNA Ab are specific for SLE (Se 60-80% and Sp 95-99%) [2,4,5]. Among the anti-ENA Ab found in SLE, anti-Sm is rare (10-30%) but highly specific for lupus (>99%) [1,2,6,7]; the other anti-ENA Ab are non-specific. These include anti-Ro/SSA, which is present in 20-30% of spontaneous lupus, subacute cutaneous lupus, and neonatal lupus; anti-U1 RNP (20- 40%); anti-La/SSB, which is rare in lupus (10-20%) and mainly seen in SLE, which begins at an extremely age [1,8]; anti-Scl70 and anti-JO-1 (10-43%). In addition to these ANA, other types of non-organ-specific auto Ab may be sought, either for differential diagnosis (as SLE is a protean disease) or because several AIDs may be associated in the same patient. These include rheumatoid factors (RF), anti-cyclic citrullinated peptide Ab (anti-CCP), anti-neutrophil cytoplasmic Ab (ANCA) and antiphospholipid Ab (aPL) [2]. The prevalence of SLE varies from 21 to 85 per 100,000 population in Europe, 11 to 103 in Asia, 18 to 178 in North America and 50 to 98 in South America [9]. In Africa, the prevalence of SLE is underestimated, with 30 cases in 10 years in Senegal (1998) [10], 16 cases in 13 years in Togo (2008) [11],11 cases in 1 year (2015) in Benin [12] and 117 cases in 27 years (2015) in Côte d'Ivoire [13]. In Côte d'Ivoire, immunological testing for SLE is standard practice. However, the high cost of this test and the variability in the specificity of auto-Ab are limiting factors for its use [11]. The aim of our study was to assess the prevalence of autoantibodies and to describe the inflammatory and immunological profile during SLE.
Materials and Methods
This is an 11-year retrospective study (from 1 January 2011 to 31 December 2021) carried out at the Immunology Unit of the Cocody University Hospital Centre, including 130 records of patients admitted for SLE, selected on the basis of the presence of a minimal inflammation and autoimmunity (ANA) work-up. The data collected related to sociodemographic (age, sex, occupation) and clinical parameters, biological inflammatory syndrome (blood count, erythrocyte sedimentation rate, C-reactive protein assay) and autoAb (ANA, native anti-DNA Ab, anti-ENA Ab, anti-CCP Ab, ANCA, anti-phospholipid Ab (aPL), rheumatoid factors). ANA was tested using the indirect immunofluorescence (IIF) technique on Hep2 cells. Anti-native DNA, anti-CCP, aPL and ANCA (anti-myeloperoxidase or anti-proteinase 3) were determined by ELISA. Anti-ENA Ab were detected by immunodot assay. RF were determined by Waaler-Rose reaction and latex test. Statistical analysis was performed with Microsoft Excel 2010.
Results
Sociodemographic data
One hundred and twenty-four patients were females and 6 males, giving a F/M sex ratio of 20.7. The mean age was 36,3 ± 10,4 years, with extremes of 12 and 67 years and a peak between 20 and 45 years (71.5%) (Figure 1).
Biological Abnormalities and Clinical Presentation
Table 1 summarises the biological and clinical manifestations. The blood count showed anaemia in 89.2% of cases, leukopenia in 42.3%, lymphopenia in 30.8% and thrombocytopenia in 23.8%. Inflammatory investigations revealed an erythrocyte sedimentation rate above 40 mm in the first hour in 92.3% of cases and a positive C-reactive protein between 8 and 51 mg/dl in 69.2% of cases. Clinical symptoms were dominated by rheumatological involvement, observed in 85.4% of patients. This consisted of arthralgia without arthritis in 51 cases (39.2%) and true arthritis in 42 cases (32.8%). Skin involvement was observed in 42.3% of cases and 37 patients (28.5%) had multivisceral lesions.
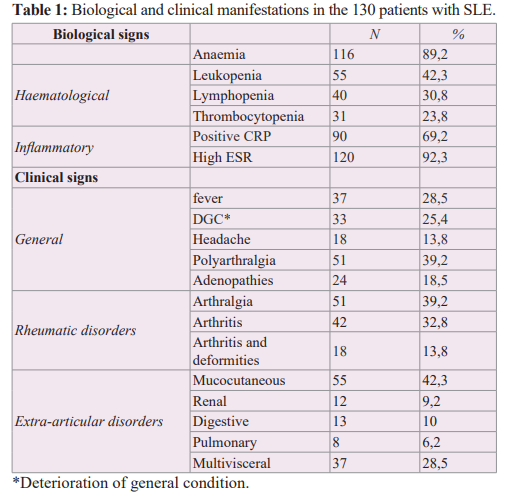
Autoantibodies
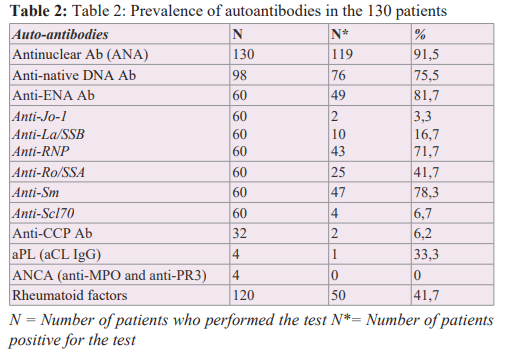
Table 2 summarises the profile of self-Ab. One hundred and six patients, or 81.5% of the cases, were ANA positive. The completion rates of native anti-DNA Ab and anti-ENA were 75.4% and 46.2%, respectively, with a positivity rate of 75.5% and 81.7%. The most common anti-ENA were anti-Sm and anti-RNP Ab with frequencies of 78.3% and 71.7%, respectively. Rheumatoid factors were performed in 92.3% and were positive in 41.7%. Anti-CCP located on the X chromosome [24], as demonstrated by the reduced prevalence of SLE in women with Turner syndrome and the significant increase in this prevalence in men with Klinefelter syndrome [24]. In SLE and other autoantibody-mediated AIDs, oestrogens appear to increase the risk of disease in genetically predisposed patients by targeting specific immunological mechanisms, including an increase in the response to type 1 interferons (IFN-α and IFN-β), differentiation of helper CD4+ T lymphocytes and survival of autoreactive B lymphocytes [4,24].
Ab were performed in 24.6% of cases and were positive in 6.2%. aPL and ANCA were rarely performed (4 cases out of 130), with 1 case positive for aPL and negative for ANCA.
Discussion
Sociodemographic Data
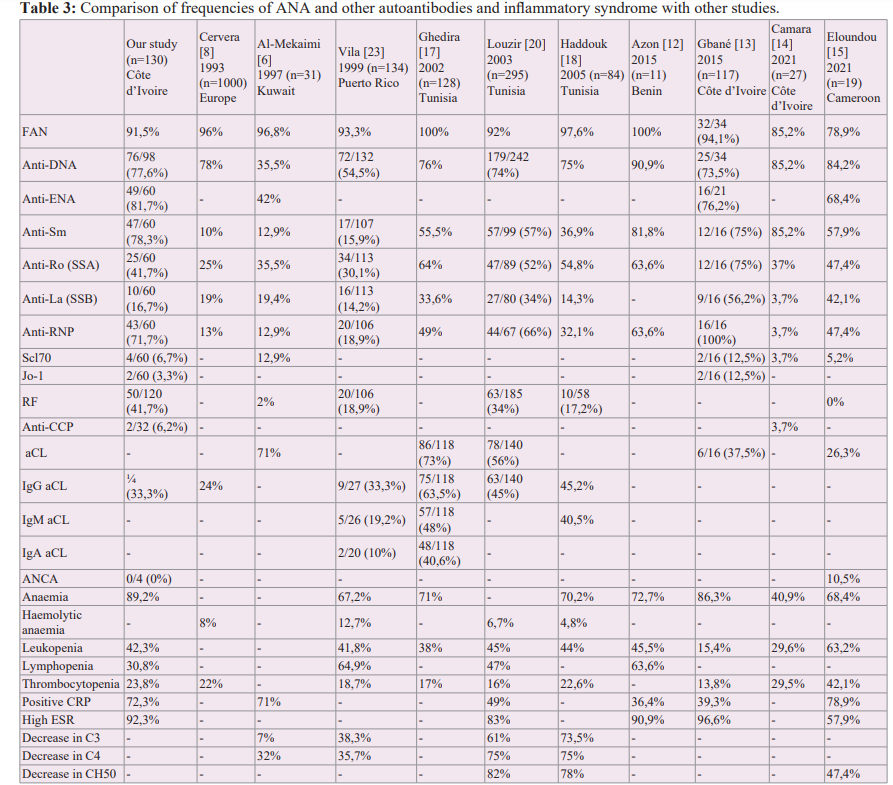
SLE is a disease of young women [11,13-21], as shown by our results, with a female-to-male sex ratio of 20.7, a mean age of 36.3 years, and a peak incidence between 20 and 45 years. The predominance of women is classic, with sex ratios ranging from 6.5 and 57.5 in black Africa [11,13-16,19,22] to 6 and 11.8 in North Africa [17,18,20]. It is 18 in South America [23], 10 in Europe [11] and 5.1 in the Middle East [6]. This supports the hypothesis that endocrine factors are involved in the pathogenesis of the disease [18,24]. Although SLE has a multifactorial origin, the sex differences observed are probably related to immunomodulation by sex hormones, but also to immunological phenomena related to pregnancy [24], as shown by the triggering role of ovulation- inducing treatments, the estroprogestogenic pill, prolactin, and pregnancy. This immunomodulation may also be due to genes
Biological Inflammatory Syndrome
The biological abnormalities observed in our series are comparable to those described in the literature [8,12-14,17,18,21-23] and are dominated by anaemia (89.2%), positive CRP (69,2%) and accelerated ESR (92,3%). Anaemia is common and is mostly inflammatory or rarely haemolytic [18,20,23,25]. The prevalence of anaemia varies in the literature from 40.9% to 72.7% [12- 14,17,18,21-23]. The anaemia is generally moderate, greater than 8 g/dL, normocytic, normochromic or, less frequently, microcytic and aregenerative. It results from 2 mechanisms: the direct effect of inflammatory cytokines (TNF-α, IFN-γ, TGF-β, IL-1 and IL- 6) on erythropoiesis [25] and cytolysis mediated by autoAb via complement activation or the ADCC phenomenon (Antibody- Dependent Cell-mediated Cytotoxicity) [26]. Cytokines act at 3 levels: direct toxicity on the early phases of erythropoiesis, insufficient production of erythropoietin and a deficit of iron available for erythropoiesis [25]. Other cytopenias are also common, most commonly of immunological origin [26,27]. Leukopenia is moderate and usually due to T-lymphopenia [26]. Thrombocytopenia is peripheral and associated with the presence of anti-platelet Ab [26]. A biological inflammatory syndrome is common in AIDs in general and in SLE in particular [6,12,13,15,19,20,22], with prevalences ranging from 39.3% to 89% for CRP [6,12-15,20,22] and from 57.9% to 100% for ESR [12,13,15,20,22]. Inflammation is mediated by pro-inflammatory cytokines, mainly TNFα, IL-1 and IL-6 [28]. IL-6 promotes the production of acute phase inflammatory proteins, particularly CRP, by hepatocytes. Often initially silent, inflammation becomes clinically significant over time and leads to local changes in cellular and tissue composition (inflammatory granuloma, tissue destruction and regrowth, fibrosis, etc.) that are difficult to normalise [29]. This inflammation is also sustained by deposition of immune complexes in the target organs and by cytolysis mediated by auto-Ab via complement activation or the ADCC phenomenon [27]. The hypocomplementemia observed in SLE in several series [6,15,18,20,23] is thought to be due to consumption of activated complement by circulating or tissue immune complexes [5,26],
although other genetic [26] or non-genetic factors may also contribute [5].
Autoantibodies
Antinuclear antibodies (ANA)
ANA are markers of non-organ specific AIDs such as SLE. Their prevalence in SLE is around 90% in black Africa [11-16,19,22], North Africa [17,18,20], South America [23], Europe [8] and the Middle East [6]. In our series, 91.5% of cases were ANA positive. ANA are also found in about 10% of healthy individuals (especially the elderly) and in many organ-specific AIDs (myasthenia gravis, multiple sclerosis, etc.) [4,27,30]. Despite this low specificity, ANA remains particularly useful in SLE due to its excellent sensitivity [5,30]. The absence of ANA makes the diagnosis highly unlikely, hence its inclusion in the diagnostic criteria for SLE established by the American College of Rheumatology (ACR) [30,31]. Finally, the absence of ANA and auto-Ab does not exclude the diagnosis of AID for several reasons: Ab may be absent initially and appear later; they may be undetectable in a patient with immunoglobulin deficiency [27]. In our study, patchy fluorescence (57.1%) and homogeneous fluorescence (26.1%) were the most common appearances on IIF. The appearance was mixed (homogeneous and speckled) in 10.9% of cases and nucleolar in 5.9%.
Native anti-DNA
High levels of anti-native DNA Ab are specific for lupus disease [3,27]. In our series, the prevalence of native anti-DNA Ab was 72.4%. This frequency is similar to the average observed in the literature, which is around 70%. [8,13,14,17-20,22]. The high positive likelihood ratio (greater than 10) shows that the measurement of this auto-Ab is very useful in confirming that a patient has SLE [7]. However, other studies report much lower frequencies, ranging from 10.6% to 54.5% [3,6,17]. These differences in frequency, particularly in Africa, may be related to the availability of serodiagnostic tests and the variable sensitivity of techniques for detecting native anti-DNA Ab. In Côte d'Ivoire, improved laboratory facilities have increased the range of tests available and led to more accurate results. Detection of native anti-DNA Ab, which used to be done by IFI on a Crithidia luciliae slide [3,6], is now done by enzyme-linked immunosorbent assay (ELISA), a more sensitive technique that improves the positivity rate of anti-DNA Ab.
Anti-ENA antibodies
In our study, the prevalence of anti-ENA was 43.07%. ENA are antigens that can be extracted from the nuclei of "collagen-rich tissue" cells. The nature of these antigens makes it possible to differentiate between collagenoses [17,32]. In the literature, the most used anti-ENA are anti-Sm, anti-Ro, anti-La and anti-RNP [6,8,12-18,20,21]. In our study, anti-Sm (77.1%) and anti-RNP (71.4%) were highly prevalent, followed by anti-Ro (42.9%). Our results are similar to those of 3 series performed in West Africa in 2015 [12-14]. Haddouk et al. found lower prevalences of anti-Sm and anti-RNP in Tunisia, in the order of 30% [18]. The different prevalences of ANA according to studies are summarised in Table III and show a large variability in the prevalence of anti-ANA depending on the population and type of study, but also on the techniques used [5]. Anti-Sm Ab are not very specific, but are highly specific for SLE [1,2,5,6,7] and are one of the biological criteria for the diagnosis of SLE. As our study and many others in the literature have shown, they are often associated with anti-U1 RNP [2,5]. Anti-RNP are not specific for SLE and are also seen in mixed connective tissue disease [5]. The frequency of anti-Ro in SLE varies from 35.5% to 75% depending on the series [6,8,12- 18,20,23]. Anti-Ro Ab are not specific for the disease but have a high predictive value for the diagnosis of SLE, especially in ANA- positive patients who do not have anti-native DNA Ab or anti-Sm Ab [18]. In SLE, the presence of anti-Ro Ab is associated with an increased incidence of photosensitivity [5,18] and with Gougerot- Sjögren's syndrome [2,5,18,27]. Anti-La Ab are rare in SLE, but when present, they are usually associated with anti-Ro Ab. Their frequency in SLE varies from 3.7% to 56.2% depending on the series [6,8,13-18,20,23].
Other autoantibodies
The prevalence of RF in our series was 41.7% (50 cases out of 120). In the literature, numerous series on SLE have reported FR frequencies ranging from 17.3% to 62.2% [13,18,22]. FR are auto-Abs, most often of IgM isotype, which recognise the Fc fragment of IgG. They are non-specific and are present in certain inflammatory conditions as well as in many AIDs, including SLE [33]. In our study, the prevalence of anti-CCP Ab was 6.2% in 32 patients.
Anti-CCP are very specific for rheumatoid arthritis (RA) (95%) and can be detected very early in the disease [33,34]. In SLE with predominant joint involvement, anti-CCP Ab may help in the differential diagnosis with RA. In addition, SLE may be associated with other connective tissue diseases, in particular RA (referred to as rhupus) [35,36], Sjögren's syndrome, antiphospholipid syndrome (APS) and systemic sclerosis (scleroplupus) [37], or other organ-specific AIDs [38]. This is known as the overlap syndrome [37].
aPL (anti-cardiolipin IgG in our study) were present in 33.3% of cases, a prevalence comparable to that reported by Gbané [13] and Vila [23] and lower than the Tunisian average: Ghedira (63.5%) [17], Haddouk (45.2%) [18] and Louzir (45.2%) [20]. aPL are pathogenic auto-Ab directed against the phospholipids of cell membranes and/ or one or more plasma or endothelial proteins associated with them (phospholipoproteins) [26]. In current practice, the aPL targeted are circulating anticoagulants (called lupus anticoagulant), anti- cardiolipin (aCL) and anti-beta 2 glycoprotein 1 (anti-β2 GP1) [26,28,39]. One of these aPL, accompanied by a clinical criterion, defines the aPL syndrome (APS). The presence of aPL in one of our patients may indicate the presence of APS associated with SLE. Several studies have shown that the presence of APS has a negative impact on the progression of lupus, not only on the occurrence of arterial thrombotic complications, but also on renal prognosis and overall survival [28]. aPL positivity is now part of the classification criteria for SLE [26]. Consequently, its dosage should be part of the systematic biological assessment in SLE.
In our series, the ANCA test results were negative. Only ANCA directed against proteinase 3 (anti-PR3 ANCA) or myeloperoxidase (anti-MPO ANCA) are clinically relevant [40,41]. Although ANCA are excellent diagnostic markers for systemic necrotizing vasculitis affecting small vessels, they are detectable in 15 to 20% of patients with SLE [41]. Eloundou in Cameroon found an ANCA prevalence of 10.5% in a study of 19 lupus patients [15].
Conclusion
Our study highlighted the presence of markers of inflammation and the variability of auto-Ab found during SLE, some of them being specific to other non-organ specific AIDs. The presence of these other auto-Ab could modify the therapeutic management and the prognosis of SLE, hence the interest in carrying out an exhaustive autoimmune assessment during SLE and systemic AIDs in general.
References
- Bardin T. Traité de thérapeutique rhumatologique. Flammarion Médecine Sciences. 2007.
- Meyer O. Devant un rhumatisme inflammatoire quels auto anticorps demander et quand. Revue du rhumatisme. 2003; 70: 803-817.
- Goran-Kouacou APV, Adou AH, Yéboah OP, et al. Prévalence des facteurs antinucléaires et des anticorps anti DNA natif chez des sujets présentant des manifestations du lupus érythémateux systémique. Revue Bio Africa. 2016; 15: 51-57.
- Fournel S, Muller S. Les autoanticorps dans le lupus. Médecine thérapeutique. 2000; 6: 537-546.
- Goetz J. Marqueurs biologiques anciens et modernes du lupus érythémateux systémique. Revue du rhumatisme. 2005; 72: 134-141.
- Al Mekaimi A, Malaviya AN, Serebour F, et al. Serological Characteristics of Systemic Lupus Erythematosus from a Hospital Based Rheumatology. Clinic in Kuwait Lupus. 1997; 6: 668-674.
- Baron Lhéritier E. Les marqueurs biologiques du lupus érythémateux disséminé intérêt des anticorps anti ficoline H. Mémoire Médecine humaine et pathologie. 2013.
- Cervera R, Khamashta MA, Font J, et al. Systemic Lupus Erythematosus Clinical and Immunological Patterns of Disease Expression in a Cohort of 1000 Patients. The European Working Party on Systemic Lupus Erythematosus Medicine. 1993; 72: 113-124.
- Pons Estel GJ, Ugarte Gil MF, Alarcón GS, et al. Epidemiology of systemic lupus erythematosus. Expert review of clinical immunology. 2017; 13: 799-814.
- Ka MM, Diallo S, Kane A, et al. Lupus érythémateux systémique au Sénégal. Médecine d’Afrique Noire. 1998; 45: 41-45.
- Kombate D, Saka B, Oniankitan OI, et al. Le lupus systémique à Lomé Togo. Médecine tropicale 2008; 68: 283-286.
- Azon Kouanou A, Agbodande KA, Kenmoe Tchouanche Wendeu Abessolo C, et al. Profil Clinique et biologique des patients lupiques suivis au centre national hospitalier et universitaire Hubert Koutoukou Maga de Cotonou. Journal de la Société de Biologie Clinique. 2015; 23: 41-50.
- Gbané KM, Ouattara B, Djaha KJM, et al. Autoantibodies in Systemic Lupus Erythematosus on Black African Subject in Abidjan. Open Journal of Rheumatology and Autoimmune Diseases. 2015; 5: 28-35.
- Camara T, Camara M, Sylla D, et al. Profil épidémiologique Clinique thérapeutique et évolutif du Lupus Systémique au service de Médecine Interne B CHU de Treichville à Abidjan. Revue Africaine de Médecine Interne. 2021; 8: 38-42.
- Eloundou P, Kamissoko AB, Ngeuleu A, et al. Profil clinique et biologique du Lupus érythémateux systémique dans un hôpital de District au Cameroun. Ann afr méd. 2021; 4269- 4274.
- Fall S, Pouye A, Ndiaye FS, et al. Présentation initiale du lupus érythémateux systémique au Sénégal. Med Afriq Noire. 2011; 5803: 156-160.
- Ghedira I, Sakly W, Jeddi M, et al. Caractéristiques cliniques et sérologiques du lupus érythémateux systémique à propos de 128 cas. Pathologie Biologie. 2002; 50: 18-24.
- Haddouk S, Ayed MB, Baklouti S, et al. Autoanticorps dans le lupus érythemateux systémique profil et corrélations cliniques. Pathologie Biologie. 2005; 53: 311-317.
- Iba Ba J, Nzenze JR, Biteghe B, et al. Profil clinique biologique et évolutif du lupus systémique en milieu hospitalier à Libreville. Médecine Afrique noire 5812. 2011; 551-559.
- Louzir B, Othmani S, Abdelhafidh NB, et al. Le lupus érythémateux systémique en Tunisie Étude multicentrique nationale À propos de 295 observations. La revue de Médecine interne. 2003; 24: 768-774.
- Zahra Haounou F, Essaadouni L Fréquence. implication clinique et valeur pronostique de la lymphopénie au cours du lupus érythémateux systémique étude cas témoin. Pan African Medical Journal. 2015; 21.
- Bija MD, Namme HL, Ashuntantang G, et al. Clinical Presentation Treatment and Outcome of Patients with Systemic Lupus Erythematosus Seen at a Rheumatology Clinic in Douala Cameroon. Health Sciences and Disease. 2014; 15: 1-5.
- Vila LM, Mayor AM, Valentín AH, et al. Clinical and Immunological Manifestations in 134 Puerto Rican patients with systemic lupus erythematosus. Lupus. 1999; 8: 279-286.
- Le Guern V. Hormones sexuelles et auto immunité. La Presse Médicale Formation. 2020; 1: 36- 41.
- Beyne Rauzy O. Anémie inflammatoire physiopathologie et prise en charge. La Revue de médecine interne. 2009; 30: S311-S314.
- Piette JC, Amoura Z, Francès C, et al. Lupus érythémateux systémique Syndrome des anti phospholipides. Rev Prat. 2003; 53: 2175-2182.
- http://www.lecofer.org/item- cours-1-15-6.php
- Godeau B. Syndrome des antiphospholipides. Hématologie.2006; 12: 101-110.
- https://www.inserm.fr/dossier/maladies-auto-immunes/
- Petitpierre S, Aubert V, Leimgruber A, et al. Utilité de la recherche des autoanticorps dans la pratique quotidienne. Rev Med Suisse. 2009; 5: 823-831.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for classification of systemic lupus erythematous. Arthritis and rheumatism. 1997; 40: 1725-1725.
- Abuaf N, Johanet C, Chretien P, et al. Detection of autoantibodies to Sm antigen in systemic lupus erythematosus by immunodiffusion ELISA and immunoblotting variability of incidence related to assays and ethnic origin of patients. Eur J Clin Invest. 1990; 20: 354-359.
- Chenevier Gobeaux C, Simonneau C, Ekindjian OG, et al. Dosage automatisé des anticorps anti peptides cycliques citrullinés sur l’analyseur Elecsys validation analytique et valeur diagnostique. Ann Biol Clin. 2009; 67: 405-410.
- Zhao Yi, Li Jing, Li Xiao Xia, et al. Anticorps anti peptides cycliques citrullinés dans le lupus érythémateux systémique. Revue du rhumatisme. 2009; 76: 873-880.
- Antonini L, Le Mauff B, Marcelli C, et al. Rhupus a systematic literature review. Autoimmunity Reviews. 2020; 19: 102612.
- Tani C, aniello D, Delle Sedie A, et al. Rhupus syndrome assessment of its prevalence and its clinical and instrumental characteristics in a prospective cohort of 103 SLE patients. Autoimmunity reviews. 2013; 12: 537-551.
- Arfa S, Saadaoui S, Brahim MB, et al. Scléro lupus forme particulière du syndrome de chevauchement à propos de huit cas. La Revue de Médecine Interne. 2023; 44: A257.
- Ghriss N, Yahia WB, Aouini C, et al. Particularités du lupus érythémateux systémique associé à d’autres maladies auto immunes. La Revue de Médecine Interne. 2020; 41: A139-A140.
- Vandana P, Anjali R, Pranaya J, et al. Prevalence of Anti Cardiolipin and Anti β2 Glycoprotein Antibodies in Indian Systemic Lupus Erythematosus Patients. International Journal of Clinical Medicine. 2011; 2: 339-345.
- Mouthon L, Millet A, Régent A, et al. Physiopathologie des vascularites ANCA positives. La Presse Médicale. 2012; 41: 996-1003.
- Sen D, Isenberg DA. Antineutrophil cytoplasmic autoantibodies in systemic lupus erythematosus. Lupus. 2003; 12: 651-658.