Proteinuria is a Key Factor Predicting the Progression of CKD: Nine- Year Follow-Up of Retrospective Health Examinee’s Cohort, Okinawa Study
Author'(s): Shoichi Mizuno1, Shaw Watanabe2*, Kunitoshi Iseki3, Chiho Iseki3 and Kozen Kinjo4
1NCC EPOC, visiting researcher, Kashiwa, Chiba, Japan.
2Tokyo University of Agriculture, Medical Rice Association,Tokyo, Japan.
3Nakamura Clinic, Urasoe, Okinawa, Japan.
4Okinawa-ken Health Promotion Foundation, Okinawa, Japan.
*Correspondence:
Shaw Watanabe, Tokyo University of Agriculture, Medical Rice Association, Tokyo, Japan.
Received: 18 Jun 2024 Accepted: 31 Jul 2024
Citation: Mizuno S, Watanabe S, Iseki K, et al. Proteinuria is a Key Factor Predicting the Progression of CKD: Nine- Year Follow-Up of Retrospective Health Examinee’s Cohort, Okinawa Study. Diabetes Complications. 2024; 8(3); 1-7.
Abstract
With the advent of an aging society, chronic kidney disease (CKD) is increasing not only in Japan but also worldwide, with 10 million patients in Japan and more than 100 million in China, India, and Southeast Asia. While low-protein diets have been effective in prolonging the life span of CKD patients, their success has not been consistent. However, this promising approach offers hope for the future. In Japan, mass health checkups have worked to prevent cancer and lifestyle-related diseases. Okinawa consists of 20 municipalities (11 on the main island and nine on remote islands), and population movement is minimal, making it easy to track changes over time.
The population of Okinawa is 1.47 million (as of October 2023), which is 1.2% of Japan's (124.64 million). The incidence of chronic dialysis patients is about 3,500 per 1 million population. The average age of dialysis patients is over 70.
In this paper, using a Cox regression model, we examined the factors leading to the end stage of CKD in a database of approximately 40,000 persons who were retrospectively followed for ten years. Nine factors significantly related to eGFR decline: 1. eGFR: estimated Glomerular Filtration Rate, 2. Cre: Creatinine, 3. UP: dipstick Urinary Protein level, 4. SBP; systolic blood pressure, 5. Hct: Hematocrit, 6.LDH: lactate dehydrogenase, 7.Urinary glucose, 8. Alb: serum Albumin, 9. FBG: Fasting Blood Glucose. The degree of urinary protein significantly advances CKD. Diabetes itself may be a risk factor for proteinuria, but the degree of glucosuria did not affect proteinuria until the late stage. Proteinuria was the essential factor in predicting CKD progression to the end stage.
Keywords
Introduction
With the reversion of Okinawa to Japan in May 1972, the Okinawa Preventive Medicine Association was established and became the Okinawa Branch of the Foundation for Parasite Prevention; regular health checkups and adult disease examinations began in 1973 and were taken over by the Okinawa General Health Association in 1992. This association, now known as the Okinawa Health Promotion Foundation, has been working tirelessly to prevent diseases in the local population by conducting health screening activities for a wide age group. Their efforts, including statutory health checkups, lifestyle-related disease prevention checkups, and various cancer checkups, are crucial in maintaining the health of the community. Currently, group health checkups are also conducted at a high rate. The total number of persons inspected in FY2022 was 774.861, with a plan achievement rate of 98.0%. The accumulated data is used for a retrospective cohort study to find the progressive factor of CKD. We decided to investigate the decline in kidney function based on nine years of health checkup data.
This time, we analyzed accumulated health checkup data in Okinawa and tried to find risk and protective factors for maintaining renal function.
Material and Method
We used health examinees’ Okinawa data accumulated from 2014 to 2022 retrospectively; the total number of health check-ups was 142,491, and a total number of participants, was 40,480 (Table 1).
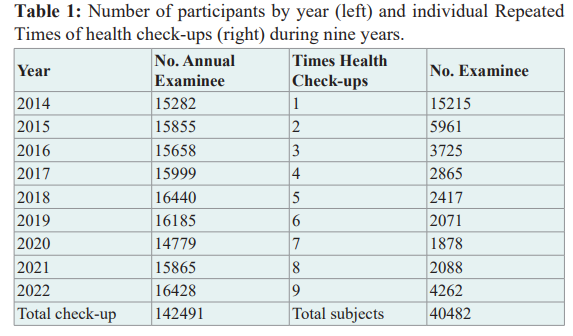
For each subject, we identified the year of the first visit as the baseline and utilized subsequent visit data prospectively. The number of variables was 27, composed of anthropometrical data, hematological and biological data, urinary data, and others. These were ID, sex, age, date of heath check-out, height, body weight, BMI, waist, blood pressure, urinary glucose, urinary protein, urinary blood, eGFR, hemoglobin (Hb), hematocrit (Hct), total cholesterol, HDL, LDL, GOT, GPT, γ-GTP, LDH, BUN, creatinine (Cre), Total protein, Albumin, Uric acid, glucose, and HbA1c.
For this study, necessary epidemiological data were downloaded to Excel files from the Okinawa Health Promotion Foundation's large server and analyzed using R statistics [1].
The Cox regression analysis (rcoxph) was applied using the minimum AIC method [2]. This method meticulously selects the essential combination of baseline factors examined in the Okinawa Health Checkup Program with an exit status of eGFR level lower than 30 ml/min/1.73m2. In some aspects, more detailed analyses were done using linear regression analysis.
Results
In the Cox regression, we looked at the data from the backward direction, based on whether or not participants reached the end stage renal disease (ESRD) at Stage 4 or 5.
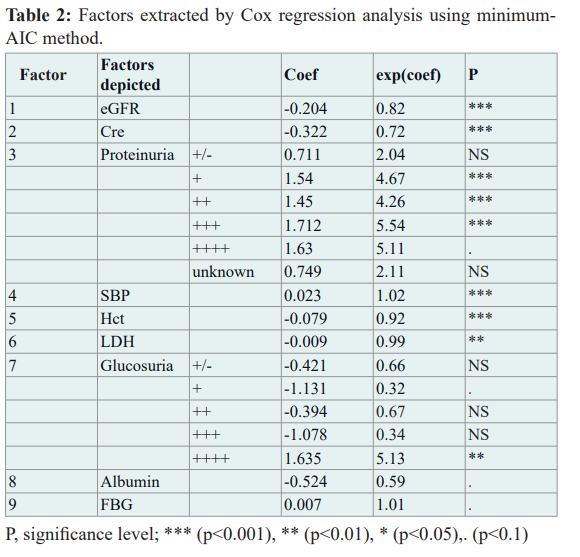
This multivariate linear Cox regression analysis identified 118 participants with eGFR levels lower than 30 ml/min/1.73m2. Nine factors were obtained (1. eGFR, 2. Cre, 3. UP: Urinary Protein level, 4. SBP, 5. Hct, 6. LDH, 7. Urinary glucose, 8. Albumin, 9. FBG: Fasting Blood Glucose) are essential for considering risk and protecting renal function. The ability of Linear Predictor (LP) to discriminate cases of eGFR≤30 ever happened was of sensitivity 91.5% and specificity 97.8% by ROC analysis. The final model's linear predictor (LP) was obtained as follows.
LP (Linear Predictor) = -0.204×eGFR-0.322×Cre + 0.711×(UP:±) +1.540×(UP: 1+ ) +1.450×(UP: 2+) + 1.712×(UP: 3+) + 1.630×(UP: 4+) + 0.023×SBP -0.079×Hct-0.009×LDH- 0.421×(US: ±) -1.131×(US: 1+ ) -0.394×(US :2+) -1.078×(US: 3+) + 1.635×(US: 4+)-0.542×Alb + 0.007×FBG.
The relationship between eGFR decline and age during the study period is shown in Figure 1.
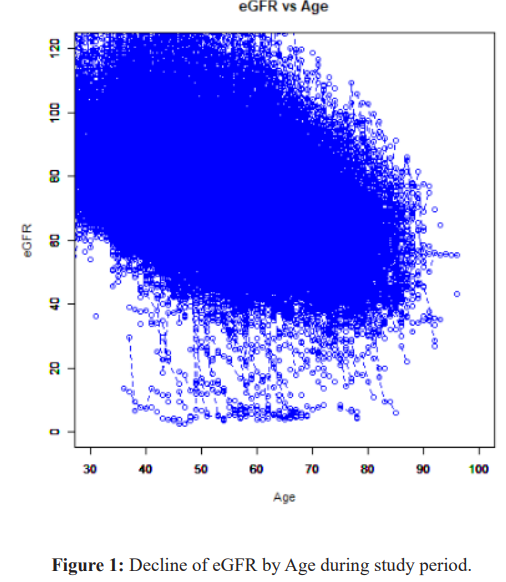
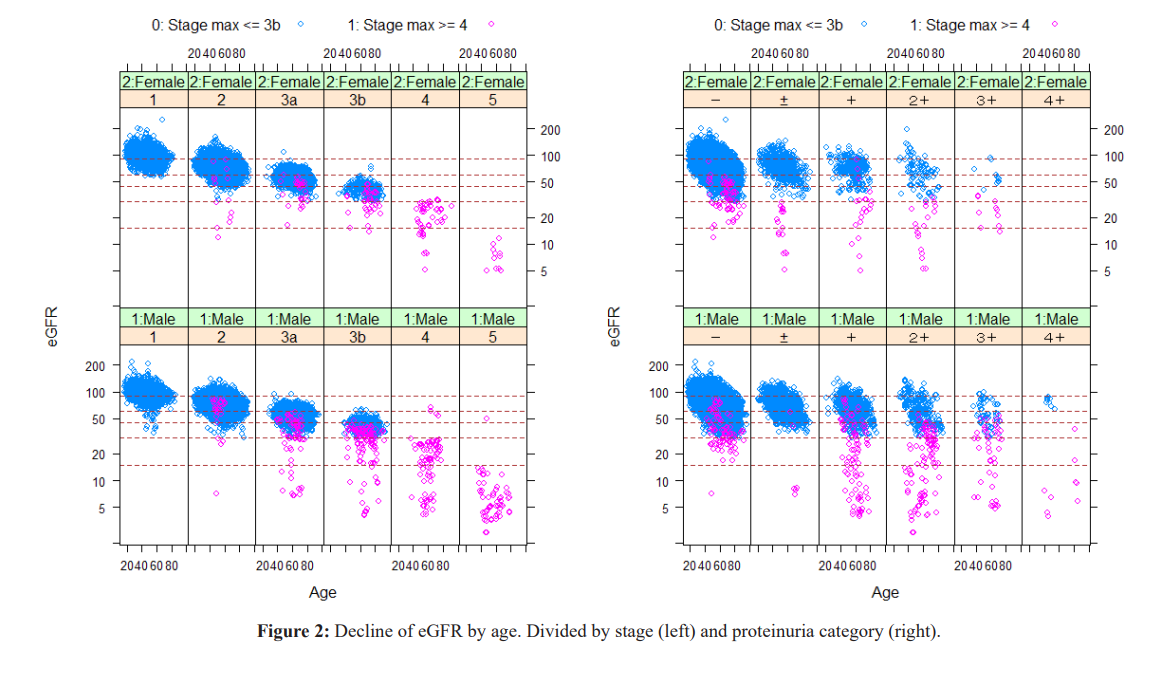
Each circle corresponds to individual participants. Stage 4 and over occurs between age 40 and 80.
In aged a slope of decline becomes slow.
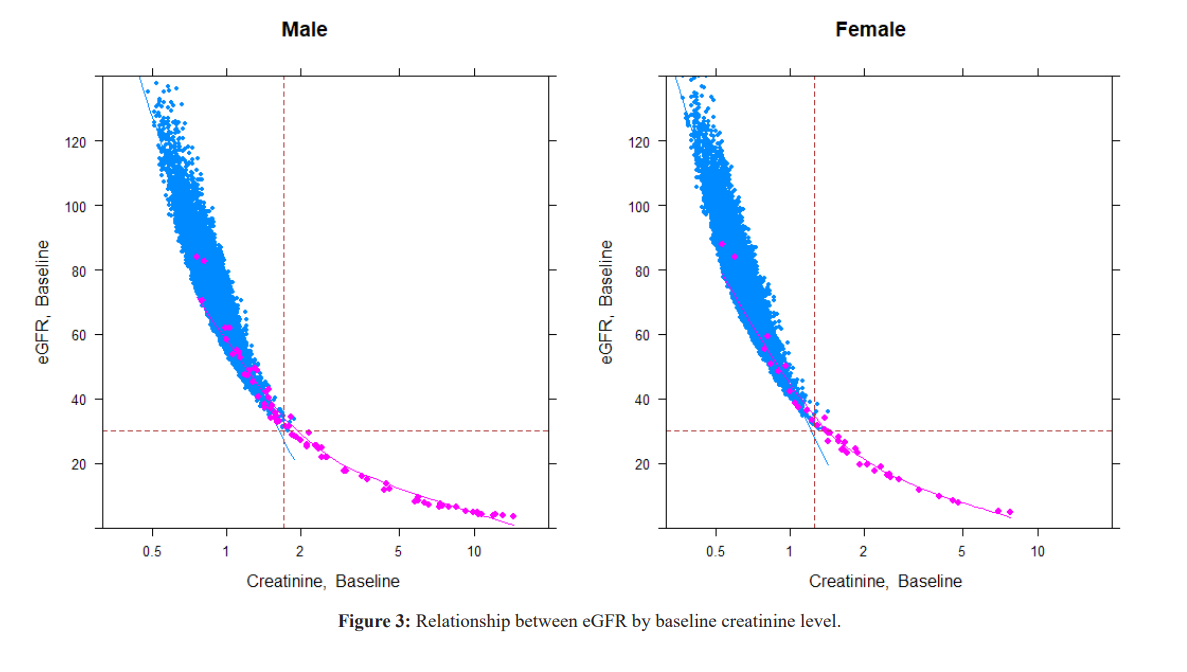
People who dropped to Stage 4 or 5 (red circle) from baseline. Prominent decrease of eGFR starts in Stage 3a and 3b, and Proteinuria (+ and ++) group, but the decline also occur from Proteinuria negative group.
The cutoff point of creatinine for decreased eGFR was 1.70 for males and 1.26 for females. The decrease in muscle mass with aging is reflected in the decrease in Cre levels in Stages 1 and 2. Cre high (reflecting muscle mass) acquired before age 40 years moderates subsequent GFR decline. The transition from Stage 3a to 3b is often observed at high Cre levels with aging.
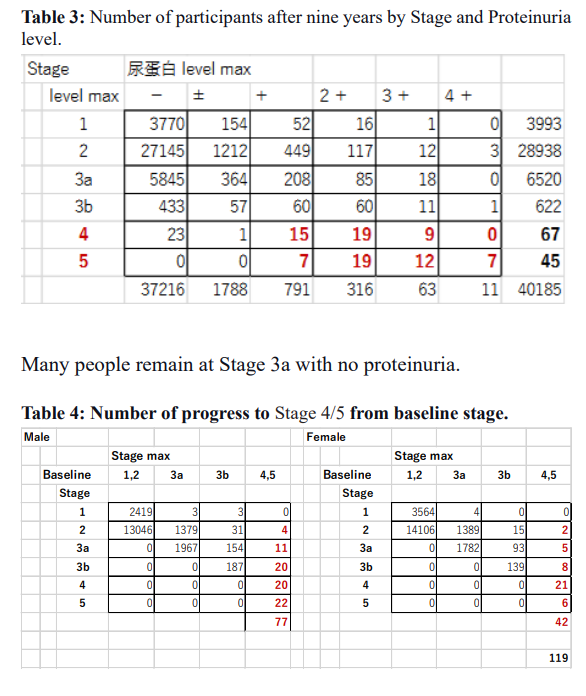
Factor 3: Proteinuria showed the essential role to progress CKD dose dependency.
Systolic blood pressure (Factor 4) correlates with age. From a causal point of view, glomerular hypertension may cause hyperfiltration, which results in proteinuria. There are many cases of slow progression around Stage 3a (3b), where the blood pressure is a little high.
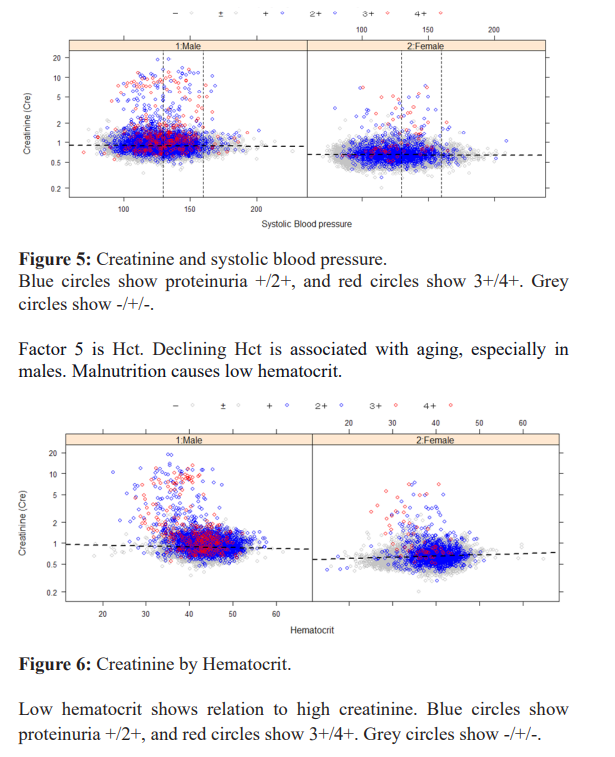
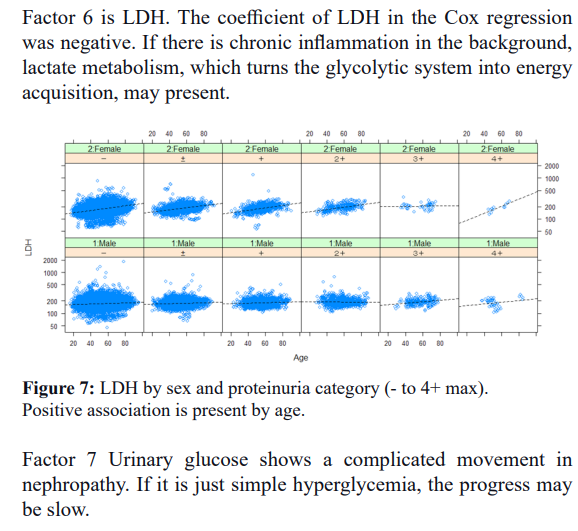
Factor 8 Alb. In the aging-related part, chronic inflammation is related, and there is a great deal of variation among individuals. Albumin decreased in malnutrition, but excretion into the urine of exhaustion in mild inflammation was also present. The A/G ratio correlates with age (r=-0.203, p<0.0001, ***); the A/G ratio is related to urinary protein+ and is a good marker of inflammation to examine its relationship with the development of chronic nephritis.
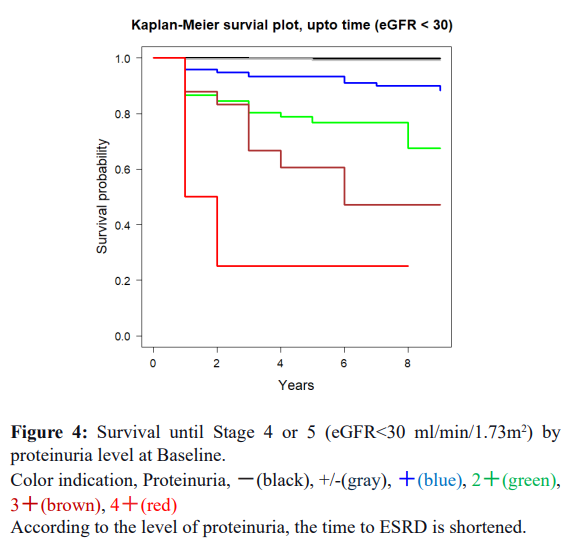
Factor 9 Blood glucose: The Cox regression result is dependent on Baseline information, so it is included as a significant factor at the Baseline. However, related factors like urinary protein work enhancers and seem to accelerate each other is multiple effects. Individual differences are significant and valuable indicators that can be used to follow individuals' progress. Protein-positive urine appears around Stage 1,2 and progresses to Stage 4/5 in a relatively short period of time (within nine years), depending on the patient. Urine glucose+ is a curious factor. It is a marker for diabetes and is somehow related to Stage, but it is not a marker for Stage progression by itself. When diabetes mellitus is combined with proteinuria, a decline in eGFR becomes apparent.
Discussion
Since either glomerular or tubular cells regenerate irreversibly in kidney disease, the goal is to slow the progression of CKD and prolong the preservation period to avoid the terminal stage of the disease [3,4]. Pharmacologic therapies such as antihypertensive agents, diuretics, and hypoglycemic agents targeting the kidneys have not reduced the number of patients on dialysis [5].
Uremia causes two conditions: uremic dysbiosis and leaky gut syndrome, in which toxins leak out due to damage to the intestinal wall epithelia, and toxins are metabolized in the liver, becoming more toxic nephrotoxins [5-7]. Breaking the negative spiral of the gut-kidney cycle is necessary to stop the progression of CKD. The close relationship between diet and intestinal microflora is essential to improve uremic toxin suppression [8,9]. Newly produced low- protein fermented genmai (Gogyo-genmai: JAS0027) is available for dietary therapy for CKD patients [10]. It guarantees low protein (<0.3g/100g boiled rice), phosphorus, and potassium while maintaining calorific value, and the dietary fiber and Y-oryzanol of brown rice components and antioxidants, which together with other functional ingredients may improve intestinal microflora and leaky gut [7].
The diet in Japan for kidney disease patients consists mainly of low-protein processed white rice or bread. It is not popular because of the bad taste. Newly made low-protein processed brown rice is preferred because of its good taste and residual functional ingredients (such as ɤ-oryzanol and dietary fiber) [7,8]. Our pilot intervention study has proven effective for various degrees of CKD [5]. Conventional ingredients meet the requirements for a long- term, continuous diet low in protein, potassium, and phosphorus but high in energy requirements. The advantage of this diet is that it can be easily applied to daily life by simply replacing the main meal with packaged rice [7]
Proteinuria is a simple and excellent marker for CKD progression. Control of proteinuria is essential to prevent CKD progression [11-15]. In 2003, Iseki et al. [15] reported the importance of proteinuria in 106,177 patients in Okinawa who were at risk of developing ESRD. Iseki et al. assessed the development of ESRD through 2000 in 106,177 screened patients (50,584 men and 55,593 women), 20 to 98 years old, in Okinawa, who participated in community-based mass screening between April 1983 and March 1984. They identified ESRD patients from the Okinawa Dialysis Study Registry. They used multivariate logistic regression analyses to calculate the adjusted odds ratio and 95% CI for the significance of proteinuria and hematuria on the risk of developing ESRD with confounding variables such as age, gender, blood pressure, and body mass index. They repeated a similar analysis in a subgroup of screened patients in whom serum creatinine data existed.
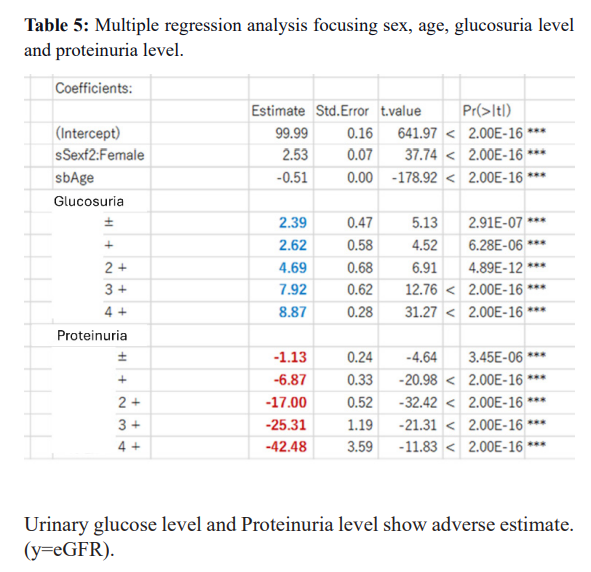
The current study repeatedly covers more factors. The advanced analyses on the same Okinawa Health Data clarified that the positive urinary protein and Cox regression results clarified that plasma due to renal insufficiency, LDH, and Albumin show the complex metabolic condition of the subjects. Progress to ESRD increased from stages 3a and 3b, corresponding to the proteinuria + and ++. Still, a small portion developed ESRD from proteinuria – and +/- groups. These people would be rapidly progressing cases within ten years.
The KDIGO CPG 2012 dietary advice [16] states: "It is recommended that CKD patients receive expert nutritional advice and information relevant to their educational program, tailored to the severity of their CKD and their intervention needs. The recommended guidelines are salt, phosphate, potassium, and protein intake. Low-protein and plant-based protein diets are the new treatment for CKD patients.
The KDOQI 2020 updated clinical practice guidelines for CKD nutrition recommend low protein diets (LPD) (0.55-0.60 g/kg/ day) or very low protein diets (0.28-0.43 g/kg/day) [16]. This is partially because previous randomized controlled trials have reported conflicting results regarding the efficacy of protein restriction on renal outcomes [17,18]. For pre-dialysis, CKD G3- G5 patients, stable metabolism, supplementation with keto acid/ amino acid analogs to reduce the risk of residual life/renal death and to improve quality of life [19]. Recently, plant-based diets high in vegetables, fruits, nuts, and whole grains, with or without minor amounts of abutments, fish, and dairy products, have been gaining attention due to their health benefits and low protein plant- based content [14,20].
A randomized trial reported that vegetarian, very low-protein diets prevented renal failure in patients with advanced CKD without increasing the risk of hyperkalemia. Several potential mechanisms have been proposed to explain why PLADO may benefit CKD patients: 1) plant-based diets may reduce intestinal- derived uremic toxins by increasing fiber intake and regulating the intestinal microflora; 2) alkaline-rich plant-based foods may prevent acidosis. 3) Plant phosphorus is bound to phytates and, therefore, has much lower bioavailability than animal phosphorus or inorganic phosphorus in food additives. 4) Plant proteins are less likely to induce glomerular hyperfiltration than animal proteins.
5) Plant-based proteins are more likely to induce hyperfiltration than animal proteins. 6) Plant-based diets increase magnesium intake. More evidence is needed to establish the efficacy, safety, and feasibility of PLADO in actual CKD patients [16,21].
There is little risk in promoting the use of plant-based diets, but the potential benefits for primary prevention of CKD and delay of progression in patients with CKD G3-5 have been suggested. These diets may also help manage and prevent some of the symptoms and metabolic complications of CKD. Recently, it has come to be said that the kidney is related to longevity through the function of the Klotho gene products and phosphate accumulation 22. The low phosphate of low-protein fermented genmai would contribute to lower phosphate levels in the body. Finally, to maintain kidney function in a healthy state, it is necessary not only to rely on the medical system while paying attention to lifestyle factors such as diet, nutritional intake, and exercise but also to cooperate and collaborate with many people in the surrounding area. Okinawa is known as an island of longevity and consists of 11 islands. Small communities have developed and have been helping each other live long lives.
Conclusion
Various factors for progressing CKD were obtained from the mass- screening program; some correlated with early stages, and others were late. Intervention to reduce depicted early or late related risks is to be planned using a systematic approach that is seamlessly and individually based. Proteinuria was the most essential factor to predict progression to end-stage renal disease. A low-protein diet effectively reduces proteinuria by improving the gut-kidney axis. Dietary intervention by plant-based food is promising. We understand that mild chronic inflammation, hypoxia, and lack of blood flow in the kidney are involved. In that case, aerobic exercise, which improves blood flow, effectively enhances metabolism.
Newly proposed KDOQI 2020 and plant-based PLADO could lead to dietary therapy shortly. Our low-protein fermented genmai is a medical food, and the effect could be evidenced by an advanced epidemiological "Pro-post comparison" design [23,24].
Acknowledgment
A part of this study was presented at the 2nd World Conference on Dietary Therapy for CKD, which was held on March 16 and 17, 2024, in Okinawa.
References
- Cryer JD, Chan KS. Time Series Analysis with Applications in Springer. 2008.
- Akaike Information theory and an extension of the maximum likelihood principle 2nd International Symposium on Information Theory BN Petrov and F Csaki eds. 1973; 267- 281.
- Watanabe Low-protein diet for the prevention of renal failure. Proc Jpn Acad Ser B. 2017; 93: 1-9.
- Ideura T, Shimazu M, Morita H, et al. Protein intake of more than 0.5 g/kg BW/Day is not effective in suppressing the progression of chronic renal failure. Contrib Nephrol. 2007; 155: 40-49.
- Watanabe S, Keika Adachi, Kunitolshi Iseki. Prevention of diabetic nephropathy by low protein processed genmai Brown Diabetes Complications. 2024; 8; 1-5.
- Vanholder R, Nigam SK, Burtey S, et al. What if not all metabolites from the uremic toxin generating pathways are toxic A Toxins. 2022; 14: 221.
- Watanabe S, Adachi K, Wakino S, et A dietary therapy with low protein genmai to improve the gut kidney axis and reduce CKD progression. Asia Pac J Clin Nutr. 2022; 31: 341-347.
- Kikuchi K, Watanabe S, Matsuo M, et Changes in microbiota and short chain fatty acids following 3 month pilot intervention study feeding brown rice balls to healthy volunteers. Prensa Med Argent. 2021; 107: 315.
- Li L, Ma L, Fu P, et al. Gut microbiota derived short chain fatty acids and kidney diseases. Drug Des Devel Ther. 2017; 11: 3531-3542.
- Watanabe S, Minakuchi S, Yamaguchi M, et al. A new low protein foodstuff from processed brown rice for chronic kidney Acta Scientific Nutritional Health. 2021; 5: 1-10.
- Katayama S, Moriya T, Tanaka S, et Japan Diabetes Complications Study Group Low transition rate from normo and low microalbuminuria to proteinuria in Japanese type 2 diabetic individuals the Japan Diabetes Complications Study. Diabetologia. 2011; 54: 1025-1031.
- Yokoyama H, Sone H, Oishi M, et Japan Diabetes Clinical Data Management Study Group Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes the Japan Diabetes Clinical Data Management study. Nephrol Dial Transplant. 2009; 24: 1212-1219.
- Araki S, Haneda M, Koya D, et Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes. 2007; 56: 1727-1730.
- Yokoyama H, Araki S, Honjo J, et al. Association between remission of macroalbuminuria and preservation of renal function in patients with type 2 diabetes with overt Diabetes Care. 2013; 36: 3227-3233.
- Iseki K, Ikemiya Y, Iseki C, et al. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003; 63: 1468-1474.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020; 98: S1-S115.
- Harn D, Hodson EM, Fouque D, et al. Low protein diets for nondiabetic adults with chronic kidney Cochrane Database Syst Rev. 2018; 10: CD001892.
- Zhu HG, Jiang ZS, Gong PY, et al. Efficacy of low protein diet for diabetic nephropathy a systematic review of randomized controlled Lipids Health Dis. 2018; 17: 141.
- Levy AS, Greene T, Beck GJ, et Dietary protein restriction and the progression of chronic renal disease What have all MDRD study results shown. J Am Soc Nephrol. 1999; 10: 2426-2439.
- Singh RB, Watanabe S, Fedacko J, et al. Dietary approaches to stop hypertension via Indo-Mediterranean Foods may be superior to DASH Diet Nutrients. 2022; 15: 46.
- Sakaguchi Y, Kaimori JY, Isaka Y. Plant-Dominant Low Protein Diet A potential alternative dietary practice for patients with chronic kidney Nutrients. 2023; 15: 1002.
- Hu MC, Kuro-oM, Moe Klotho and kidney disease. J Nephrol. 2010; 23: S136-S144.
- Watanabe Medical Foods Beyond the functional food claim. Acta Scientific Nutritional Health 2024; 8: 37-40.
- Watanabe S. A Trap of RCT in low protein dietary therapy. Revaluation of Pro-Post Study for dietary intervention. Acta Scientific Nutritional 2023; 7: 35-38.