Radiotherapy in Extramammary Paget’s disease: It’s worth it? A Case Report and Literature Review
Author'(s): Ilaria Benevento1, Grazia Lazzari1*, Giuseppe Trojano2 , Antonella Bianculli3, Antonietta Montagna1, Barbara D’Andrea1, Raffaele Tucciariello3, Giovanni Castaldo1, Francesca Sanseverino4 and Angela Pia Solazzo1
1Radiation Oncology Unit, IRCCS, CROB, Rionero in Vulture(PZ), Italy.
2Gynecology Oncology Unit, Madonna delle Grazie Hospital, Matera (MT), Italy
3Physic Unit, Radiation Oncology Unit, IRCCS, CROB, Rionero in Vulture (PZ), Italy.
4Gynecology Oncology Unit, IRCCS, CROB, Rionero in Vulture(PZ), Italy.
*Correspondence:
Grazia Lazzari, MD, Radiation Oncologist, Radiation Oncology Unit, IRCCS -CROB -Rionero in Vulture (PZ), Italy.
Received: 24 Sep 2023; Accepted: 31 Oct 2023; Published: 07 Nov 2023
Citation: Ilaria Benevento, Grazia Lazzari, Giuseppe Trojano, et al. Radiotherapy in Extramammary Paget’s disease: It’s worth it? A Case Report and Literature Review. Cancer Sci Res. 2023; 6(1): 1-5.
Abstract
Extramammary Paget’s disease (EMPD) is a rare and heterogeneous clinical picture usually recorded on apocrine gland-bearing epidermis areas in extramammary sites, showing a potential invasive evolution and poor prognosis. At diagnosis, it could appear as a primary tumor with secondary co-exhisting visceral malignancies like in anus, male or female genital system, which should be assessed. This rare epidermal carcinoma generally occurs in individuals older than 60 years mainly in Caucasian female and Asian male ethnicity. Although the pathophisiology has been well established, its management is still controversial and requires a multidisciplinary team approach. There are several therapeutic strategies. Among them, radiotherapy is accounted as an effective option in primary or in adjuvant timing but up to now no definitive role has been assessed. Data by case series, case reports and retrospective analyses have provided results on the positive benefit of HDR brachytherapy or external beam radiotherapy (EBRT) mainly in very old patients not suitable for extended surgery, which remains the main treatment care. Long course radiotherapy is a big concern in elderly patients due to logistics and care assistance deficiencies, which makes their access difficult to the radiation centres. Thus, hypofractionated radiotherapy regimens could be a good compromise problem solving to provide excellent local control within a short treatment time. Herein, we present a case of EMPD occurring in a 80 years old woman with vulvo-perineal extension successfully treated with hypofractionated external beam radiotherapy as primary treatment using a schedule 300cGy/fr/51 Gy in 17 treatment days.
Keywords
Introduction
The term “Extramammary Paget’s disease” (EMPD) refers to an epidermis cancer arising outside the breast nipple-areolar skin area [1]. This disease shows two typical findings: a) it occurs in apocrine gland-bearing no breast skin areas with an invasive evolution and visceral involvement; b) it is typical in the older population over 60 years [2,3]. In a Chinese study conducted in 2016, its crude prevalence has been estimated 0.4 per 1,000,000 people [4]. In Europe, the crude incidence rate and age standardized incidence rate 0.7 and 0.6 per 1,000,000 person-years, respectively has been found in a study conducted in 2012 [5]. In regard to gender distribution, it seems that in Caucasian populations, EMPD has a female predominance, with male-to-female ratios ranging from 1:2 to 1:7; in Asian population, EMPD shows a male predominance with a male to female ratio 3.5:1 [6,7].
At initial presentation, the differential diagnosis of EMPD from contact dermatitis, lichen sclerosis, psoriasis or mycosis fungoides needs to be done due to its clinical features similar to skin inflammatory diseases. In fact it appears like persistent erythematous or eczematous plaques not responding to common topic therapies evolving into lichenification, bleeding or deep ulceration [8].
Pruritis or pain associated with skin irritation and burning are the most common symptoms. Moreover, clinically palpable and enlarged regional nodes may also be present as expression of metastases [9]. Co-existing visceral or genital malignancies associated with this entity have been reported in more than 25%- 35% with anus, vagina, vulva, scrotum as the main common involved sites [6]. This involvement could be as a primary or a secondary manifestation related to EPMD, although the differential diagnosis between primary or secondary malignancies is very difficult to assess. EPMD usually involves the epidermis but it may progress into a ca in situ or develop an invasive pattern, yielding a very poor prognosis [10]. Thus, when a EMPD diagnosis occurs, a co-existing occult malignancies should be suspected and assessed. Punchy biopsy or mapping biopsy are mandatory for diagnosis; factors like delay in diagnosis, depth of invasion > 1 mm, lymphovascular invasion and node metastases at diagnosis are poor prognosticators [9].
Taken together these factors have been grouped into a TNM staging system proposed by Ohara et al. which could be useful in the therapeutic making decision [11]. Several mechanisms within genetic altered pathways seem to be involved in its pathogenesis leading to novel medical approaches [12]. However, surgery and radiotherapy are the most accounted therapeutic strategies. Local excision with wide clear margins of normal skin of 2-5 cm of normal skin followed by reconstruction are required [13], although limiting the extent of resection is mandatory for functional sparing and cosmetic outcomes, especially in cases of urethra or anus involvement. Moreover patients are usually very old with comorbidities which make them not suitable for extended surgery.
Radiotherapy is reported as the first-choice alternative therapy to surgery as in primary as in, adjuvant approach, but to date, information is limited to sufficently establish its curative role in this disease. Going through the literature data, it has been demonstrated that HDR brachytherapy or external beam radiotherapy provide a long term local control and good cosmetic outcome. Experiences on patients treated with HDR- brachytherapy Kilovoltage and electrons-photons radiotherapy have been reported assuming that median total doses of ≥ 60 Gy delivered with conventional fractionation in median 49 days are safe and effective [14]. Hypofractionated schedules have been also adopted in several cases. Herein we report the case of an 80 years old woman complaining of EMPD of vulvo-perineal area successfully treated with hypofractionated radiotherapy 300 cGy x 17 fractions to 51 Gy total dose.
Case Presentation
Informed consent was obtained from the patient to publish this report. An 80-year-old woman went to our observation complaining of painful burning with pruritus and extended skin erythema to the vulvar and perineum region since 4-6 months before. Her medical history was negative for allergy, diabetes, drug assumption. Lesion took up a wide area and showed a butterfly morphology with lichenifications and confluent eczematous plaques with initial ulceration (Figure 1). A mapping biopsy on perineal skin and vulvar region showed the histopathological diagnosis of EPMD confined only to epidermis. Handheld reflectance confocal microscopy (HRCM) confirmed no deep invasion. Colonscopy and gynecological examination were performed to assess an underlying visceral malignancies. No visceral involvement was found. CT scan of pelvis and perineum showed any nodal involvement so the stage was T0 N0M0 according to Ohara proposal classification. Due to the extent of lesion and advanced age, wide perineal skin surgery was not allowed. Surgeon sent her to our observation asking for a palliative radiotherapy. Moreover, the patient was frail, she had no care giver and lived more than 100 km from a radiation center unit. Once assessed that radiotherapy is an effective alternative to surgery, on behalf of the patient’s difficulties, we decided to prescribe a hypofractionated schedule. According to ASTRO clinical practice guidelines for external beam radiotherapy skin cancer [15], we choose a hypofractionated regimen consisting of 300 cGy x 17 fractions to 51 Gy total dose which corresponds to 66.3 Gy in BED10. Treatment was delivered with VMAT using a 5 mm thickness bolus and 6 MV photon beams. As recommended by clinical practice guidelines by Kibby et al. [16], clinical target volume (CTV) consisted of the involved vulvar and perineal skin plus margin 3 cm to encompass the entire vulvar and perineal visible skin disease. Planning target volume (PTV) included CTV plus a 1 cm margin. On external, PTV was cropped 5 mm under the bolus to ensure the dose to the skin. Hot-spot below the 105 % of the PD were accepted. (Figure 2) Patients fully completed the treatment without interruptions. As acute side effect she developed skin erithema and dysuria. After 6 months of follow up the patient remains in complete remission, the perineal skin appears burned but renewaled (Figure 3).
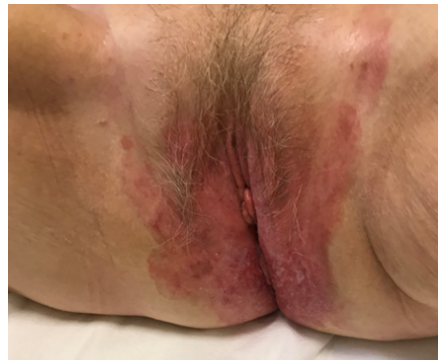
Figure 1: Image of pretreatment clinical presentation.
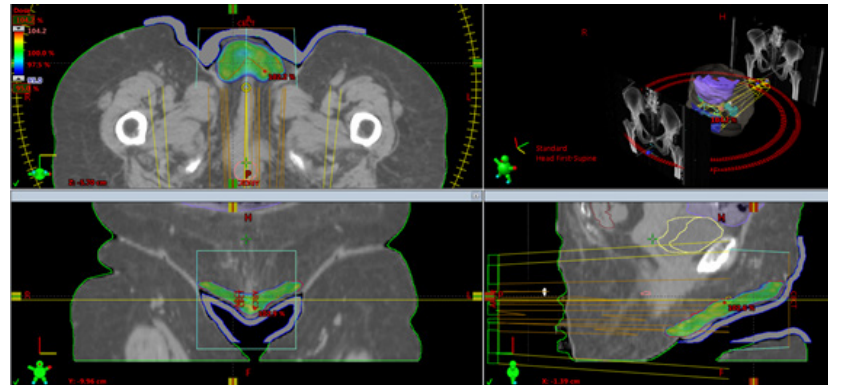
Figure 2: Treatment plan with dose distribution in VMAT with bolus.
Thus, anti-target therapies are under investigation [22].

Figure 3: The clinical outcome after 6 months off therapy.
Discussion
EMPD was first described by Crocker in 1888 [17]. Over time, it has been well aknowledged that EMPD is a rare disorder which frequently occurs in the the anogenital areas and affects specifically population over 60 years old, women and male according the geographic prevalence [4,5,7]. Its origin has been hypothesized from pluripotent keratinocytes stem cells or intraepidermal cells of the apocrine gland ducts as confirmed by the presence of folliculo- sebaceus-apocrine units in anogenital regions [2]. Pathogenesis accounts for disorders in P16 protein expression not related to HPV or ERB-B2 gene altered amplification [18,19]. Altered pathways of RAS and PIK3CA are also evocated [20]. Moreover altered androgen receptors signalings seem to be involved [21].
Medical topical therapies, surgery and radiotherapy are the common adopted treatments. Surgery remains the cornerstone requiring excision with wide clear margins followed by reconstruction [13]. However local recurrence is accounted due to a high rate of positive margins ranging from 36-65% [23,24]. Then adjuvant radiotherapy may follow [25]. Old and frail patients may be not amenable to surgery thus primary radiotherapy as an alternative to surgery should be offered. This is the most accepted field of application as represented in the infographic picture by Tolia et on 57 invasive EMPD patients treated with radiotherapy. As a result, Japan and China have been found (older population factor) as the most involved countries using radiotherapy, accounting for 44 and 15 cases respectively, with a mean age of treated patients of 71.8 years and the mean diameter of irradiated lesions nearly 10.9cm [26]. Which techniques to apply doesn’t a matter. Experiences with HDR and external beam radiotherapy have been reported by over 20 years, both of them showing favourable outcomes and long term disease control. Among HDR brachytherapy series, first Kwan et al. reported on a patient with scrotal EPMD treated with fractionated HDR brachytherapy on a customed multicatheter wax mould. Patient received 42 Gy in 14 fractions over 18 days and recorded a complete regression. Epitheliosis and disuria were the acute reported side effects [27]. In the report of Carrozzo et al., five patients with ano-genital EPMD were treated with 188- Re -Dermo-Beta-Brachytherapy. As a result, all treated patients showed a complete clinical and pathological regression [28].
More recently, Risso S et al. showed a case of a patient with scrotal EPMD with positive margins after hemiscrotectomy, treated with HDR with a H.A.M. applicator and CT treatment planning. At five years follow up the patient is still disease free [29]. Concerning the role of external beam radiotherapy, data from several case reports and case series agree on its long term efficacy as primary or adjuvant approach. A first report in 1991 by Brierley on six patients with EPMD of genitalia and perineal area treated with RT 3000-5400 cGy, showed a long term disease control [30].
Subsequently cases by other authors and papers on hundreds of treated patients have demonstrated how doses of 40-60 Gy could be effective and able to ensure a long term control with negligible acute side effects [31,32]. Standard fractionation seems the main preferred regimen as confirmed by Hata’s report [33]. The authors indicated a median total dose to the gross tumor volume (GTV) nearly 60 Gy (45-80.2) in 23-43 fractions (frs) with 1.8 / 2.2 Gy/ fr within a median overall treatment of 49 days (31 - 69 days) as the most used schedule. Overall, studies have been collected and summarized within a recent systematic review by Kibbi et al. [16] By this systematic review and analysis on 483 studies conducted through a multidisciplinary expert panel evaluation, clinical practice guidelines have been developed, giving information on doses, fractionations, fields sizes, and techniques [16]. Radiotherapy has resulted as the primary treatment modality in 7.5% of cases (263 of 3507 cases) with a recurrence rate of 30.6% (11of 36 studies). Adjuvant radiotherapy in 8.5% of cases (296 of 3466) with a recurrence rate of 34.8% have been recorded. These guidelines suggest primary radiotherapy as a curative option with Grade B and category 2 evidences specially in case of inadvisable or unfeasible surgery while adjuvant radiotherapy is advised in case of persistent or recurrent as Grade C and category 2.
Data on doses provide values ranging from 30 to 64 Gy in 20 to 33 fractions in primary setting while in adjuvant approach 50 Gy median doses (range from 45 to 64.8Gy) to tumoral bed and median dose 60Gy (59-70 Gy) on lymphonodes are recommended. Radiation treatment fields sizes should account for subclinical extension with fields limits extending 3.5 cm beyond the clinical border to encompass the all the visible tumor in order to limit the injury to adjacent tissues like anus or vagina. The decision to include the draining nodal should be individualized on the basis of risks factors. In regard with altered fractionation, several retrospective studies encourage the use of hypofractionation. In the study of Luk, six patients were treated with curative radiotherapy, using several different schedules with electrons or photons (33 Gy/10 frs, 60 Gy / 27-28 frs, 32 Gy /8 frs) assuming the biological effective dose (BED) for late-reacting normal tissues with α/β ratio 3, as calculated by the linear quadratic model from 69 Gy3 to 118 Gy3. These BED values were considered equivalent to standard fractionation total to a total dose of 42–70 Gy using 2 Gy-fractions. regimens. Results were effectve [34].
Yanagi et al. described two cases sussessfully treated with hypofractionated radiotherapy. Both patients were women older than 80 years with EPMD on the vulvar skin. One patient was treated with an electron beam of 4 MeV, 2.25 Gy per day, 4 days⁄ week to a total dose of 45 Gy; the other one was treated with X-rays of 4 MV, 2.5 Gy per day, 4 days ⁄ week to a total dose of 45 Gy. An electron beam therapy as boost (4 MeV, 3.0 Gy per day, for 5 fractions to a total dose of 15 Gy) was added only to thick lesions on both sides of the genitals. A complete response was achieved in both of them with dermatitis as the most important acute side effect. The clinical outcome was a complete remission as confirmed by the histology on the irradiated specimens [35]. Tackenberg reported results on seven older patients, six of them receiving radiotherapy with 20 to 30 kV electrons at doses of 200 to 400 cGy per day for 2 to 5 days per week unto a total dose of 4000 to 5600 cGy. One patient was treated with a radiograph of 40 kV at 400 cGy per day for 2 days per week until a total dose of 4800 cGy. The most commonly reported side effect was mild dermatitis with reddening and desquamation. Initially all patients obtained a complete durable remission in almost of all them [36]. Our experience on this patient treated with hypofractionated external beam radiotherapy and curative intent according to ASTRO recommendations is in line with the literature data and published clinical practice guidelines [15].
Conclusions
Given the rarity of this disease, its incidence in elderly and the growing ageing of population worlwide, curative radiotherapy (EBRT or HDR) is a valid approach to offer. Moreover, hypofractionated radiotherapy is safe and effective and constitutes a good compromise between patient care logistic issues and quality of life.
References
- Lloyd J, Flanagan Mammary and extramammary Paget’s disease. J Clin Pathol. 2000; 53: 742-749.
- Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985; 13: 1009-1014.
- Asel M, Leboeuf NR. Extramammary Paget’s disease. Hematol Oncol Clin North Am. 2019; 33: 73-85.
- Ghazawi FM, Iga N, Tanaka R, et al. Demographic and clinical characteristics of extramammary Paget’s disease patients in Japan from 2000 to 2019. J. Eur. Dermatol. Venereol. 2021; 35: e133-e135.
- van der Zwan JM, Siesling S, Blokx WA, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur. J. Surg. Oncol. 2012; 38: 214-221.
- Funaro D, Krasny M, Lam C, et al. Extramammary Paget disease: Epidemiology and association to cancer in a Quebec- based J. Low. Genit. Tract Dis. 2013; 17: 167-174.
- Cheng PS, Lu CL, Cheng CL, et al. Significant male predisposition in extramammary Paget disease: A nationwide population-based study in Br. J. Dermatol. 2014; 171: 191-193.
- Preti M, Micheletti L, Borella F, et Vulvar Paget’s disease and stromal invasion: Clinico-pathological features and survival outcomes. Surg. Oncol. 2021; 38: 101581.
- Iwamoto K, Nakamura Y, Fujisawa Y, et al. Depigmented extramammary Paget’s disease without histological dermal invasion identified by multiple inguinal and pelvic lymph node metastases. Eur. J. Dermatol. 2018; 28: 387-388.
- Scarbrough CA, Vrable A, Carr DR. Definition, Association with Malignancy, Biologic Behavior, and Treatment of Ectopic Extramammary Paget’s Disease: A Review of the Literature. J. Clin. Aesthet. Dermatol. 2019; 12: 40-44.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: Retrospective analysis of 301 patients with invasive primary tumors. J. Dermatol. Sci. 2016; 83: 234-239.
- Kang Z, Xu F, Zhang QA, et al. Oncogenic mutations in extramammary Paget’s disease and their clinical relevance. Int. J. Cancer. 2013; 132: 824-831.
- Chung PH, Leong JY, Voelzke BB. Surgical Experience With Genital and Perineal Extramammary Paget’s Disease. Urology. 2019; 128: 90-95.
- Ishizuki S, Nakamura Y. Extramammary Paget’s Disease: Diagnosis, Pathogenesis, and Treatment with Focus on Recent Developments. Curr. Oncol. 2021; 28: 2969-2986.
- Likhacheva A, Awan M, Barker CA, et al. Definitive and postoperative radiation therapy for basal and squamous cell cancers of the skin: executive summary of an American Society for Pract Radiat Oncol. 2020; 10: 8-20.
- Kibbi N, Owen JL, Worley B, et al. Evidence-Based Clinical Practice Guidelines for Extramammary Paget JAMA Oncology. 2022; 8: 618-628.
- Crocker Paget's disease affecting the scrotum and penis.Trans Pathol Soc London. 1889; 40: 187-191.
- Al-Obaidy KI, Kao CS, Idrees MT. P16 expression in extramammary Paget’s disease of the vulva and scrotum is not human papilloma virus Int Surg Pathol. 2018; 26: 617-620.
- Tanaka R, Sasajima Y, Tsuda H, et al. Human Epidermal Growth factor receptor 2 protein over expression and gene amplification in extramammary Paget’s disease. Br J Dermatol. 2013; 168: 1259-1266.
- Kang Z, Xu F, Zhang QA, et al. Oncogenic mutations in extramammary Paget’s disease and their clinical relevance. Int J Cancer. 2013; 132: 824-831.
- Azmahani A, Nakamura Y, Ozawa Y, et Androgen receptor, androgen-producing enzymes and their trascriptions factors in extramammary Paget’s disease. Hum Pathol. 2015; 46: 1662-1669.
- Fukuda K, Funakoshi T. Metastatic extramammary Paget’s disease: pathogenesis and novel therapeuthic Front Oncol. 2018; 8: 38-41.
- Edey KA, Allan E, Murdoch JB, et al. Interventions for the treatment of Paget’s disease of the Cochrane Database Syst. Rev. 2019; 6: Cd009245.
- Murata Y, Kumano K. Extramammary Paget’s disease of the genitalia with clinically clear margins can be adequately resected with 1 cm margin. Eur J Dermatol. 2005; 15: 168-170.
- Hata M, Koike I, Wada H, et al. Postoperative radiation therapy for extramammary Paget’s disease. Br. J. Dermatol. 2015; 172: 1014-1020.
- Tolia M, Tsoukalas N, Sofoudis C, et Primary extramammary invasive Paget’s vulvar disease: what is the standard, what are the challenges and what is the future for radiotherapy?. BMC Cancer .2016; 16: 563.
- Kwan WH, Teo ML, Yu KH, et Perineal Paget’s disease: effective treatment with fractionated high dose rate brachytherapy. Clin Oncol. 1995; 7: 400-401.
- Carrozzo AM, Cipriani C, Donati P, et al. Dermo Beta brachytherapy with 188Re in extramammary Paget’s G Ital Dermatol Venereol. 2014; 149: 115-121.
- Risso S, Amendola BE, Perez NC, et al. High-dose-rate brachytherapy in scrotal extramammary Paget's disease: A case report. Brachytherapy. 2023; 22: 210-213.
- Brierley JD, Stockdale AD. Radiotherapy: an effective treatment for extramammary Paget's disease. Clin Oncol. 1991; 3: 3-5.
- Besa P, Rich TA, Delclos L, et al. Extramammary Paget's disease of the perineal skin: role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992; 24: 73-78.
- Burrows NP, Jones DH, Hudson PM, et al. Treatment of extramammary Paget's disease by Br J Dermatol. 1995; 132: 970-972.
- Hata M, Koike I, Wada H, et al. Radiation therapy for extramammary Paget’s disease: Treatment outcomes and prognostic factors. Ann. Oncol. 2014; 25: 291-297.
- Luk NM, Yu KH, Yeung WK, et al. Extramammary Paget’s disease: outcome of radiotherapy with curative intent. Clin Exp Dermatol. 2003; 28: 360-363.
- Tackenberg S, Gehrig A, Dummer R, et al. External Beam Radiotherapy of Extramammary Paget Disease. Cutis. 2015; 95: 109-112.
- Yanagi T, Kato N, Yamane N, et al. Radiotherapy for extramammary Paget’s disease: histopathological findings after radiotherapy. Clinical and Experimental Dermatology. 2007; 32: 506-508.