Results of A Multicentric Study of Patients with Multiple Myeloma Treated with Three Different Triplet Regimens Including Bortezomib
Author'(s): Alvarado Ibarra Martha1, Mujica Martínez Aldo1, Pérez Zuñiga Juan Manuel2, Hernández Ruiz Eleazar3, Paredes Lozano Eugenia Patricia4, Trejo Gómora Jorge5, Ron Guerrero Carlos6, Alvarez Vera José Luis1, Ortiz Zepeda Maricela1 , Mena Zepeda Veronica1, De la Peña Celaya Antonio1, Espitia Rios Eugenia1,Ramírez Romero Fabiola3, Resendíz Olea Rodrigo2 and López Hernández Manuel1
1 Hematology Department, Centro Médico Nacional “20 de Noviembre”, Ciudad de México, ISSSTE, México.
2 Hematology Department, Hospital Regional Ignacio Zaragoza, Ciudad de México, ISSSTE, México.
3 Hematology Department, Hospital Regional Presidente Juárez,Oaxaca, Oaxaca, ISSSTE, México.
4 Hematology Department, Hospital Regional 1 de Octubre, Ciudad de México, ISSSTE, México.
5 Hematology Department, Hospital Regional Adolfo López Mateos, Ciudad de México, ISSSTE, México.
6Hematology Department, General Hospital "Aquiles Calles Ramìrez", ISSSTE Tepic, Nayarit, Mexico.
*Correspondence:
Martha Alvarado Ibarra, Hematology Department, Centro Médico Nacional “20 de Noviembre”, Ciudad de México, ISSSTE, México, E-mail: normoblasto@gmail.com.
Received: 30 July 2018; Accepted: 01 September 2018
Citation: Alvarado Ibarra Martha, Mujica Martínez Aldo, Pérez Zuñiga Jua Manuel, et al. Results of A Multicentric Study of Patients with Multiple Myeloma Treated with Three Different Triplet Regimens Including Bortezomib. Cancer Sci Res. 2018; 1(3): 1-8.
Abstract
Background: Chemotherapy with multiple drugs has been a crucial step in the treatment of Multiple Myeloma producing a high deep response rate and improving progression-free survival and overall survival. The efficacy of bortezomib in the induction of remission has been demonstrated in different studies. Cytotoxic drugs such as Doxorubicin, Cyclophosphamide or Thalidomide have been combined with Bortezomib / Dexamethasone. There are few randomized studies comparing remission with Bortezomib / Dexamethasone with Cyclophosphamide, Doxorubicin or Thalidomide inductions treatments.
Primary Endpoints: Primary Endpoints were to compare Overall Survival (OS), Progression-Free Survival (PFS) and Response Rates. As Secondary Endpoints, to determine the factors that correlate with PFS and to know the incidence of adverse events.
Patients and Methods: A prospective, multicenter, comparative cohort study including patients diagnosed with multiple myeloma who received Bortezomib-Dexamethasone plus Cyclophosphamide (BORCIC) or Doxorubicin (BORDOX) or Thalidomide (BORTAL) as the first-line chemotherapy. Evaluated and compared data were: response, progression-free survival, overall survival and toxicity.
Results: A total of 201 patients were studied from 2010 to 2015, 88 women (44%), 113 men (56%). The characteristics of the disease were similar for each treatment group. Distribution of monoclonal component type was 20% IgA (40), 62% IgG (125), nonsecretory and light chains 18% (36). Durie-Salmon Staging was: I 17% (35), II 33% (67), III 50% (99). Staging by International Staging System (ISS) was I: 18% (n = 37), II: 44% (n = 90) and III 38% (n = 74). 40% (80) of patients started with a fracture at the time of diagnosis. Renal failure was present in 20% (40) of the patients at diagnosis. The overall response: Group (BORCIC) CR/VGPR (n= 60) was 87%; Group (BORDOX) CR/VGPR (n=44) was 69% and in Group (BORTAL) CR/VGPR was 91% (n=63); p 0.006. Progression was greater in group (BORDOX, n = 36), compared with group (BORCIC, n = 27) and group (BORTAL, n = 23) (p 0.04). Median progression-free survival for group (BORTAL) was 36 months, for group (BORCIC) was 28 months and for group (BORDOX) was 20 months (p 0.006). Median OS for those with CR was not reached, for those with VGPR was 27 months and for those with PR was 17 months (p 0.0001). The overall incidence of neuropathy was BORCIC n=46, BORDOX n = 39, BORTAL n = 47, (p 0.77).
Conclusion: In our experience, the best first-line treatment regimens for patients with multiple myeloma are those that include Bortezomib, Dexamethasone with Thalidomide or Cyclophosphamide, with no difference in response rate and response type, overall survival and progression-free survival. These regimens showed similar toxicity rates. The regimen that includes Doxorubicin is the regimen with the worst results for both progression-free survival, overall survival and toxicity, so we do not suggest it as a first-line treatment regimen.
Keywords
Introduction
Currently, the agents with more effects on progression-free survival and overall survival are the combinations of different drugs such as Thalidomide, Bortezomib, Alkylating agents (Cyclophosphamide), Anthracyclines (Doxorubicin) and more recently second generation immunomodulators (Lenalidomide) and the new Proteosome Inhibitors [1-5].
This combination of regimens in the induction of remission improves the overall response and the depth of the response by increasing the percentage of patients who achieved complete response (CR) [6-8]. However, the initial therapy in MM depends on risk stratification, patient conditions and availability of resources. Therefore, numerous regimens have been tested for the treatment of MM, separating those patients that are candidates from those patients who are not candidates for AHCT because progression-free survival and overall survival are better [9-12].
For now, it is well known the superiority of regimens containing Bortezomib-Dexamethasone. The data supporting their uses come from single-arm trials or from randomized studies that compare these regimens with others that are not considered as first-line [13- 15].
Some comparisons have been made with regimens containing Bortezomib plus Dexamethasone with Thalidomide (VTD) or with Cyclophosphamide (VCD) where it has been reported after 4 cycles of treatment that 66% of patients with VTD had at least VGPR vs. 56.2% for VCD arm (P = 0.05), and with better impact on Overall Response with VTD arm of 92.3% vs 83.4% in VCD arm (P = 0.01) [16-20].
For adults older than 65 years, regimens have been compared in retrospective studies. For example, in a study in which the results of patients in this age with Melphalan-Prednisone -Thalidomide (MPT) regimens were compared vs. Bortezomib-Dexamethasone- Doxorubicin with a PFS of 80 months in the MPT group and in the VAD group median was not reached (P = 0.03) [21-25].
There are no studies so far that compare the different Bortezomib- based treatment regimens in Mexico and even in Latin America.
Endpoints
Primary Endpoints were to compare Overall Survival (OS), Progression-Free Survival (PFS) and Response Rates.
As Secondary Endpoints, to determine the factors that affect PFS and to know the incidence of adverse events.
Patients and Methods
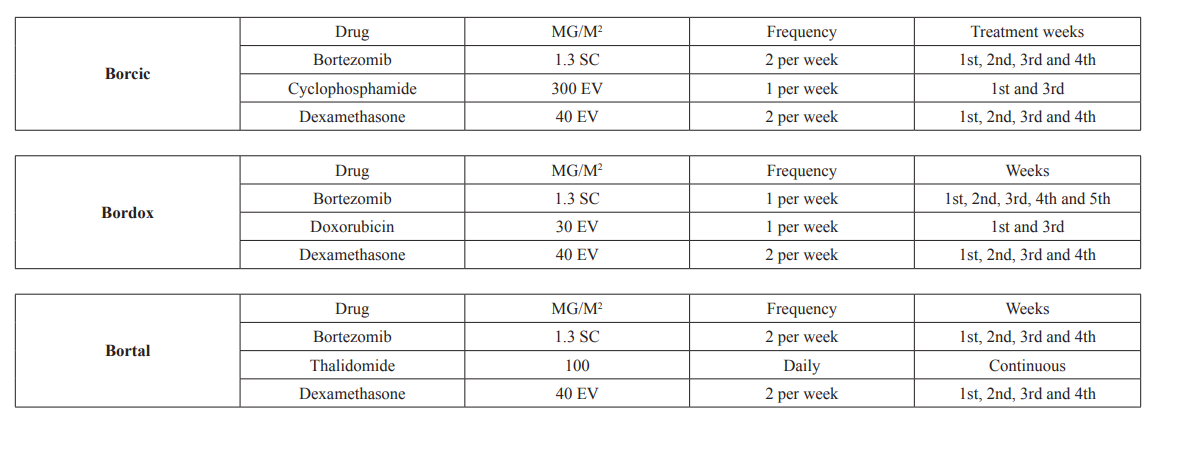
An experimental, longitudinal, multicentric, prospective cohort study. Patients older than 18 years, of either sex, were included, regardless of previous comorbidities with a recent diagnosis of Multiple Myeloma by Hematology Unit of the following Hospitals: Servicio de Hematología del Centro Médico Nacional 20 de Noviembre, Hospital Regional Ignacio Zaragoza, Hospital Regional Presidente Juárez de Oaxaca, Hospital Regional 1 de Octubre, Hospital Regional López Mateos y Hospital General Tepic Nayarit; all these Hospitals are from ISSSTE (Social Security Institute for Federal Government Employees) according to WHO criteria established in 2008 and to the International Myeloma Group 2014 criteria (Table 1 and 2 in annexes) and that received at least 4 treatment cycles of one of the following regimens: GROUP BORCIC: Bortezomib, Dexamethasone, Cyclophosphamide, GROUP BORDOX: Bortezomib, Dexamethasone, Doxorubicin, GROUP BORTAL: Bortezomib, Dexamethasone, Thalidomide (see Treatment Groups in Annexes), Exclusion criteria: patients with previous chemotherapy regimens and/or who do not have complete data in clinical and electronic file.
Discontinuation criteria: patients who have had a lack of adherence to the current Hospital treatment protocols, to die before the fourth cycle of treatment, to withdraw treatment or those who were lost during follow-up.
Chemotherapy was applied as follows.
Each cycle consisted of 28 days. For all groups the Bortezomib dose was 1.3 mg/m2 intravenously or subcutaneously twice a week on days 1, 4, 8, 11, 15, 18, 22 and 25 of each cycle and 40 mg Dexamethasone intravenously on the same days as Bortezomib. For group (BORCIC), Cyclophosphamide 300 mg/m2 i.v. on day 1, and 22. For group (BORDOX) total dose of Doxorubicin was 30 mg and for group (BORTAL) Thalidomide was 100 m / day throughout the cycle. Bortezomib treatment used an i.v. route of administration most of the times, the analyzed variables were Sex, Age, Hemoglobin, Platelets, Calcium, Creatinine, Albumin, B2 microglobulin, Lactic Dehydrogenase, C-reactive protein, ECOG, Durie-Salmon Stage, ISS (International Staging Index ), Treatment scheme are explained in tables 5, 6 and 7 of annexes], Renal Insufficiency, Immunoglobulin Type, Adverse Events, Progression-Free Survival, Overall Survival (See definitions in table of annexes).
The primary objective was to know and compare the response to treatment, overall survival, progression-free survival, and as secondary objectives to determine if there are prognostic factors that have an impact on progression-free survival by treatment group and to know the toxicity by treatment regimen.
The information was obtained from clinical files, electronic records and follow-up sheets. This study was approved by the Centro Médico Nacional 20 de Noviembre Hospital Research Committee, number 338.2017, which is subject to Declaration of Helsinki guidelines and Guide on Research Involving Human Subjects, guaranteeing respect for the following principles: Beneficence, Autonomy, Non-maleficence and Justice. And according to General Law of Health, article 100, chapter V. This investigation did not exist, Researchers or Institutions were not interested.
Statistical Analysis
Nominal variables were shown in percent, numerical variables were shown in mean, median, minimum, maximum and standard deviation. t-student test was used to compare numerical variables and they were corroborated with Kruskal-Wallis test and ANOVA table. Chi-square test was used to compare nominal variables and they were corroborated by Pearson's chi-squared test.
Descriptive analysis was performed with measures of central tendency and dispersion, absolute measures and percentages according to the type of variable. Measures of association and statistical significance (risk rates and p-value), were used for a univariate analysis with obtained data and its association with progression was evaluated.
Search for prognostic data was performed with ANOVA, Chi- square and t-student tests. Linear regression was used for the univariate and multivariate analysis. OS, PFS were calculated with the Kaplan-Meier nonparametric method as the median survival in months, an overall survival curve was made.
Statistical significance was considered with a p<0.05 value. To carry out the statistical tests and to obtain the survival curves, the statistical program SPSS 23.0 (SPSS Inc. Chicago, Illinois, USA) was used.
Results
From January 2010 to 2015, 201 patients were included, 88 women (44%), 113 men (56%) older than 18 years, of any sex, regardless of previous comorbidities. Demographic characteristics by treatment group were balanced. The proportion according to its functional state was, ECOG <2 =150 patients (75%) y ECOG >2 = 50 patients (25%). Bence-Jones was positive in 82 (41%), negative in 119 (59%). Distribution of monoclonal component was 40 patients type IgA (20%) , type IgG 124 patients (62%), nonsecretory and light chains 37 (18%). Durie-Salmon Staging was: staging I, 35 patients (17%), staging II, 69 (34%) and staging III, 97 (49%). Staging by International Staging System (ISS) was : I in 36 patients (18%), II in 80 (39%), III in 85 (42%). Of the total number of patients, 80 (40%) started with a fracture at the time of diagnosis. Renal failure was present in 40 (20%) of the patients at diagnosis.
Chemotherapy rate was: BORTAL 69 patients, 34 women, 35 men; BORDOX in 63 patients, 24 women, 39 men; BORCIC in 69 patients, 28 women, 41 men (p 0.5). Mean age for each regimen was: BORTAL 57 years, BORDOX and BORCIC 56 years (p 0.14). Mean Hemoglobin was 10.5 g-dl, platelets 257 mil, albumin 2.8 g-l (0.9-4.6), Beta 2 microglobulin 2.7 (0.4-2.8) mg-l, Lactic Dehydrogenase 154 U-l, C-reactive Protein 6 mg/dl y Creatinine 0.9 mg-dl. Distribution of these variables was similar in each group (Table 1).
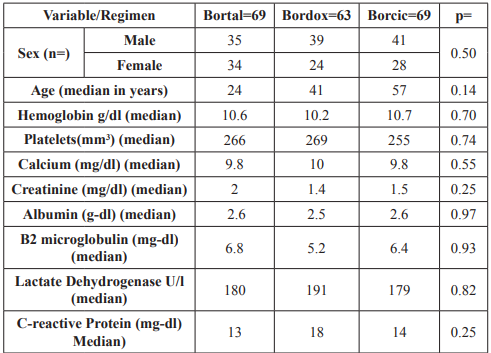
Table 1: General results: Lactate Dehydrogenasea.
The distribution by Durie-Salmon, immunoglobulin type, ISS, was practically similar between groups.
The overall response rate in the following was found for group BORCIC with CR/VGPR in 87%; in group BORDOX: with CR/VGPR in 69%; and in group BORTAL with CR/VGPR in 92% ,being similar between group BORTAL and BORCIC but significantly different from group BORDOX (p= 0.006). Progression was greater in group 2 (BORDOX), n = 36), compared with group 1 (BORCIC, n = 27) and group 3 (BORTAL, n = 10), while this last group has shown to be the best regimen in this item (p 0.04) Table 2.
There were 13 deaths in group BORCIC, 38 deaths in group
BORDOX and 10 deaths in group BORTAL (p 0.001). Of the causes of death, 25 were due to Infections, 20 due to Renal Insufficiency, 10 due to Diabetes Mellitus, 3 due to Acute Myocardial Infarction and 3 due to other causes (Unknown) Table 3.
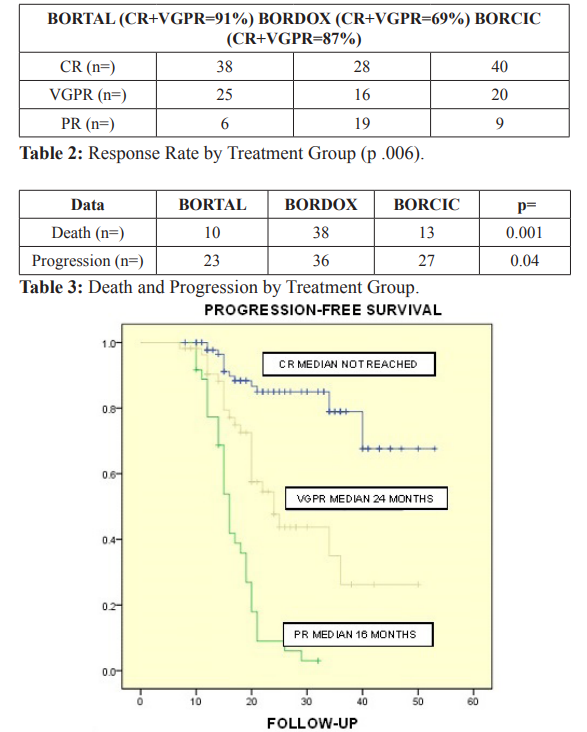
Figure 1: Progression-Free Survival was assessed according to the degrees of response achieved, finding superiority for those who achieved Complete Response, with an unreached median. For those who obtained Very Good Partial Response, the median was 24 months and for Partial Response (PR) it was 16 months (p 0.001).

Figure 2: In Progression-Free Survival analysis by treatment group, superiority was also found for group BORTAL, reaching a median of 36 months, followed by group BORCIC with a median of 28 months and finally group BORDOX with a median of 20 months (p 0.006).
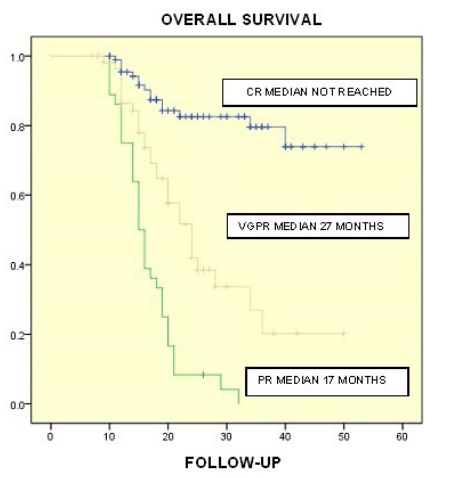
Figure 3: Median OS according to response level was not achieved for those with CR, for those with VGPR was 27 months and for those with PR was 17 months (p 0.0001).
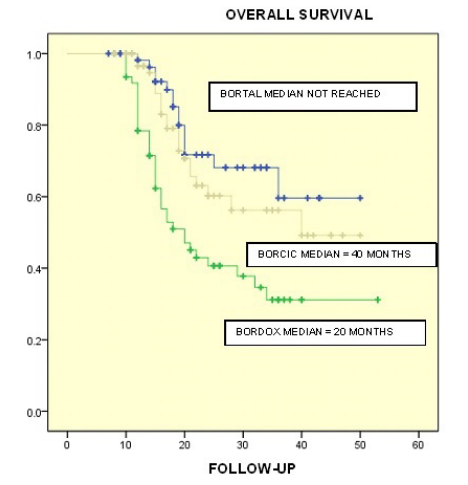
Figure 4: Differences were found in Overall Survival per Treatment Group. For group BORTAL OS was not reached, for group BORCIC a median of 40 months was found while for group BORDOX the median was 20 months in a follow-up period of 50 months (p = 0.0001).
In the univariate analysis it was found that high Durie-Salmon Stage and elevated LDH (Lactate Dehydrogenasea) represent adverse prognostic factors for progression-free survival, (HR 1.9, 95% CI, 1.1-3.1, p 0.005 and HR 1, 95% CI, 0.9-1.1, p 0.02, respectively). In the multivariate analysis, the Durie-Salmon Stage maintained its impact as a prognostic factor, (HR 1.7, 95% CI, 0.8- 2.6, p 0.015) while DHL remained the predictor (HR 1, 95% CI, 0.9-1.3; p 0.034) Table 4.
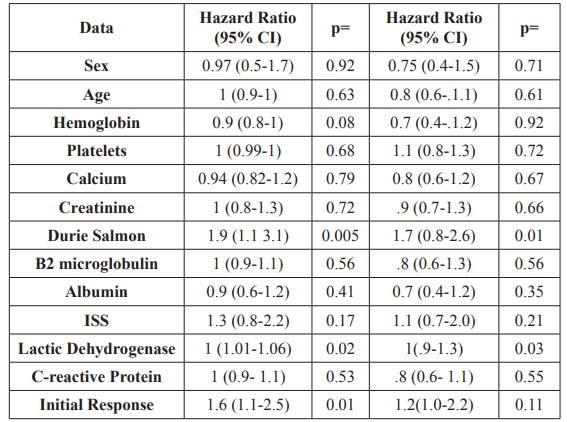
Table 4: Univariate and multivariate analysis: Progression Free Survival.
As for toxicity, an overall incidence of neuropathy of 66% (132) was found. In the analysis by group, no significance was found BORCIC, n = 46, BORDOX n = 39, BORTAL n = 47, p 0.77).
Neuropathy Grade 3-4 was higher in BORTAL arm (n=34) in comparison with BORDOX (n= 17) and BORCIC (n=19). (p=0.02). An overall incidence of Herpes Zoster of 12% (n= 24), with no differences found by treatment group (p 0.42).
Discussion
In this multicenter prospective cohort study, 3 different triplet bortezomib-based chemotherapies were analyzed. Patients who had completed at least 4 treatment cycles and who have the necessary data to assess the disease were analyzed.
Treatment groups were named by the name they receive in the Procedures Manual of the Hematology Service of that Medical Center. Baseline characteristics of each group were very similar. We emphasize that for Cyclophosphamide regimen, it is administered intravenously, unlike other regimens (CyBORD for example) because ISSSTE (Social Security Institute for Federal Government Employees) has no oral cyclophosphamide.
As expected, the immunoglobulin type with the highest prevalence was type G, followed by type A and non-secretory type in which the light chains subgroup was included.
An incidence of renal failure similar to that reported in other groups of 21% was found. In these reports, a correlation of subjects with renal failure at diagnosis with early mortality of 19.5 versus 40.4 months was found [26,27].
However, these reports are before the advent of Bortezomib. After the advent of Bortezomib, overall survival in patients with renal failure was compared in retrospective studies, finding lower overall survival in those with glomerular filtration rates lower than 60 ml/ min/m2, but no statistical significance was found [28]. We did not find an impact on progression-free survival when patients started the study with renal failure. This is attributable to the benefit of the drug in this population.
Despite the fact that most patients had in advanced ISS stages and a high tumor burden by Durie-Salmon Stage, this did not imply an impact on progression rate in the multivariate analysis. It is essential to explain that the above mentioned is attributable to the fact that the time to start the treatment is late, allowing more advanced stages to be established, but without having an impact on the incidence of renal failure. We did not find that renal failure was a prognostic factor in PFS and OS, perhaps because most patients had resolution during treatment.
As for Response to Treatment, comparisons with study drugs additional to Bortezomib, there are reports with Bortezomib, Dexamethasone, Thalidomide (VTD) vs. Bortezomib, Dexamethasone, Cyclophosphamide (VCD) in patients with recent Myeloma diagnosis and as an induction treatment prior to transplantation. This group found that the VTD combination increased more than 3 times the complete response rate compared to VCD (19 vs. 6%, p 0.001). Similarly, overall response rates were higher in the VTD arm than in the VCD arm [14,30].
We did not have primary refractoriness in studied patients, finding some type of response in all of them. The regimens with the best response (CR+VGPR were included here) was the regimen with Thalidomide (BORTAL), followed by the combination with Cyclophosphamide (BORCIC) and finally with Doxorubicin, which contrasts with some other groups that compared the Cyclophosphamide group vs. Doxorubicin, without finding differences in response rates [16]. However, among the group of Thalidomide (BORTAL) and Cyclophosphamide (BORCIC) a minimal difference was found in favor of the BORTAL group with respect to overall response rates.
In our analysed population, we found that factors with prognostic impact, with significant risk rates for progression-free survival and overall survival, were the type of achieved response with a non-achieved mean for those subjects with Complete Response. Although, generally, the treatment arm was not relevant while a complete response was reached, the benefit was derived from the Thalidomide and Cyclophosphamide arms. In summary, because most of the patients who achieved this type of response were in the group with Thalidomide followed by the group with Cyclophosphamide, these had a greater impact on the Progression- Free Survival and Overall Survival.
Although most of these patients had advanced ISS stages and a high tumor burden, a large proportion of them had a functional status by ECOG equal to or less than 2, so we did not find a direct association between these factors.
Some studies, such as phase III HOVON-65/GMMG-HD4, compare the Vincristine, Doxorubicin and Dexamethasone (VAD) regimen vs. Bortezomib, Doxorubicin and Dexamethasone (PAD), finding greater progression-free survival with PAD, from 13 to 30 months in high-risk patients [11]. However, there are few direct comparisons in survival between different Bortezomib- based regimens, some are with drugs such as Lenalidomide vs. Cyclophosphamide [29].
It is noteworthy that the overall rate of neuropathy was higher than in other groups, in which the reported incidence of neuropathy was 7% to 22% for the VTD combination compared to 1% to 13% for VCD [14,30].
The highest rates of neuropathy were in the BORTAL arm. In the analysis by Level of Neuropathy, the severity (grades 3 and 4) was higher in the combination with Thalidomide that justified the adjustment in the doses without affecting the responses in this treatment group. We concluded that this higher toxicity rate was because, Bortezomib was administered intravenously in our first patients.
The incidence of Herpes Zoster even with Acyclovir prophylaxis was similar to that reported by other groups. There was no difference by treatment group, corroborating in our universe of patients that the risk factor for the development of this infection is due to Bortezomib, without affecting the rest of the drugs.
As for mortality rates, these were greater with the regimen that included Doxorubicin, with significance with respect to the other two regimens in which the rates were similar. This led to the discontinuation of BORDOX regimen from our treatment protocols. On the other hand, in addition to the above, the highest rates of progression also were within the BORDOX regimen, suggesting that mortality in this treatment regimen was due to complications of the disease or comorbidities such as Diabetes Mellitus, Ischemic Heart Disease and other unspecified conditions, but not due to adverse drug events.
In Mexico due to financial issues in Public Health there is less availability of Bortezomib for the treatment of Multiple Myeloma. The records that evaluate the drug in our population are null, so the interest arose to report our results for these three drug-based regimens and to extend the scope to the authorities of our country.
Conclusion
In our experience, the best first-line treatment regimens for patients with multiple myeloma are those that include Bortezomib, Dexamethasone with Thalidomide or Cyclophosphamide, with no difference in response rate and response type, overall survival, progression-free survival and with Similar Toxicity Rates. The regimen that includes Doxorubicin is the regimen with the worst results for this item, so we do not suggest it as a first-line treatment regimen. On the other hand, we suggest the regimens that include Thalidomide as a first-line treatment due to the improved rates of progression-free survival despite there are no greater differences in response rates compared with the cyclophosphamide-based regimens.
ANNEXES
Definition of variables
Progression-Free Survival: The length of time during and after the treatment of a disease, such as cancer, that a patient lives with the disease but it does not get worse.
Complete Response: Serum or urine immunofixation negative, disappearance of plasmocytoma in soft tissues and ≤ 5% of plasma cells in bone marrow.
Very Good Partial Response: Detection of serum and urine M-protein by immunofixation but not by electrophoresis or 90% or higher reduction of M-protein, plus reduction <100mg/24h of M-protein in urine.
Partial Response: ≥50% reduction of serum M-protein, and ≥90% or <200mg/24h reduction of urine M-protein. ≥50% reduction in the size of soft tissue plasmacytomas.
Stable Disease: It does not meet the previous criteria, nor those of progression.
Disease Progression: ≥25% Increase of baseline serum and urine M-protein, ≥10% increase of plasma cells in Bone Marrow.
Development of new bone lesions or soft tissue plasmacytomas or increase in their size, hypercalcemia.
Chemotherapy toxicity: Adverse effect associated with chemotherapy drugs.
BORCIC: Chemotherapy that includes Bortezomib, Cyclophosphamide and Dexamethasone according to CMN 20 November Hospital Protocol.
BORDOX: Chemotherapy that includes Bortezomib, Doxorubicin and Dexamethasone according to CMN 20 November Hospital Protocol.
BORTAL: Chemotherapy that includes Bortezomib, Thalidomide and Dexamethasone according to CMN 20 November Hospital Protocol.
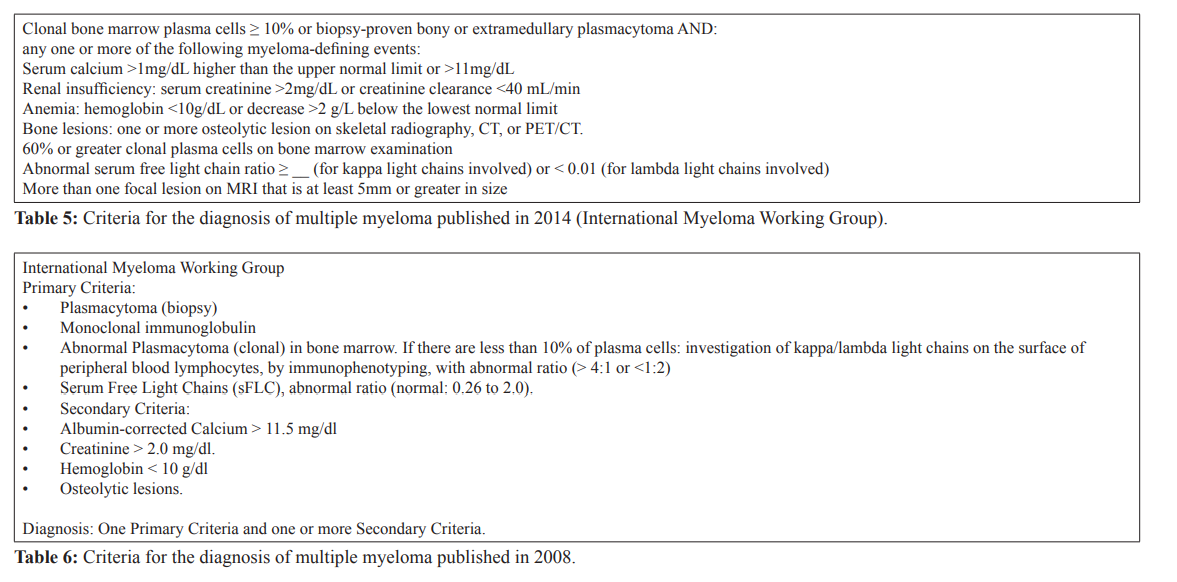
Multiple Myeloma: Hematologic neoplasia based on the International Myeloma Working Group criteria for the diagnosis of multiple myeloma published in 2014.
References
- Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011; 183: 25-35.
- Cornell RF, Kassim Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: increased options and increased complexity. Bone Marrow Transplantation. 2016; 51: 479-491.
- Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008; 111: 2516-2520.
- Alexanian R, Haut A, Khan AU, et al. Treatment for multiple myeloma: combination chemotherapy with different melphalan dose regimens. JAMA. 1969; 208: 1680-1685.
- Anderson H, Scarffe JH, Ranson M, et VAD chemotherapy as remission induction for multiple myeloma. Brislih Journi d Cancer. 1995; 71: 326-330.
- Rajkumar SV, Kumar S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clin Proc. 2016; 91: 101-119.
- Palumbo A, Cavallo F, Gay F, et Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014; 371: 895.
- Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012; 119: 4375-4382.
- Rosinol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012; 120: 1589-1596.
- Sonneveld P, Goldschmidt H, Rosinol L, et al. Bortezomib- based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013; 31: 3279-3287.
- Sonneveld P, Schmidt-Wolf IGH, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 J Clin Oncol. 2012; 30: 2946-2955.
- Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010; 28: 4621-4629.
- Cavo M, Tacchetti P, Patriarca Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2011; 378: 1846.
- Philippe Moreau, Cyrille Hulin, Margaret VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016; 127: 2569-2574.
- Alvarado-Ibarra M, Briones-Cerecero R. Resultados de tres esquemas de tratamiento en pacientes con mieloma múltiple mayores de 65 años. Rev Hematol Mex. 2016; 17: 90-98.
- Mai EK, Bertsch U, Dürig J, et Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma Leukemia. 2015; 29: 1721-1729.
- Khan ML, Reeder CB, Kumar SK. Comparison of lenalidomide/dexamethasone versus cyclophosphamide/ lenalidomide/dexamethasone versus cyclophosphamide/ bortezomib/dexamethasone in newly diagnosed multiple myeloma. Br J Haematol. 2012; 156: 326-333.
- Rebecca Solomon, Alberto Gabizon. Clinical Pharmacology of Liposomal Anthracyclines: Pharmacology of Liposomal Anthracyclines: Focus on Pegylated Liposomal Doxorubicin. Clin Lymphoma Myeloma. 2008; 8: 21-32.
- Rami Manochakian, Kena C. Miller, Asher Alban Chanan- Khan. Bortezomib in Combination with Pegylated Liposomal Doxorubicin for the Treatment of Multiple Myeloma. Clin Lymphoma Myeloma. 2007; 7: 266-271.
- Craig B. Reeder, Donna E. Reece, Vishal Kukreti, et al. (CyBorD) induction for newly diagnosed multiple myeloma: High response rates in a phase II clinical trial. Leukemia. 2009; 23: 1337-1341.
- Faith E. Davies, Ping Wu. The combination of cyclophosphamide, velcade and dexamethasone (CVD) induces high response rates with comparable toxicity to velcade alone (V) and velcade plus dexamethasone (VD); Haematologica. 2007; 92: 1149-1150.
- Kropff M, Bisping G. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol. 2007; 138 :330-337.
- Harousseau JL, Nagler A. Effect of the combination of pegylated liposomal doxorubicin and bortezomib on time to progression (TTP) and overall survival of patients with relapsed/refractory multiple myeloma compared with bortezomib alone. J Clin Oncol; 2007; 25: 8002.
- Voorhees PM, Dees EC, O'Neil B, et al. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003; 9: 6316-
- Antonio Palumbo, Kenneth Multiple Myeloma. N Engl J Med. 2011; 364: 1046-1060.
- Eleutherakis-Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic Leuk Lymphoma. 2007; 48: 337-341.
- Yang G, Chen W, Wu Y. Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment. Chin Cancer Sci Res. 2013; 25: 155-160.
- Isik Kaygusuz, Tayfur Toptas, Fergun Bortezomib in patients with renal impairment. Hematology. 2011; 16: 200-208.
- Dimopoulos MA, Beksac M, Benboubker Phase II study of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide for sub-optimal response as second-line treatment for patients with multiple myeloma. Haematologica. 2013; 98: 1264-1272.
- Cavo M, Pantani L, Pezzi A, et al. Bortezomib-thalidomide- dexamethasone (VTD) is superior to bortezomib- cyclophosphamide-dexamethasone (VCD) as induction therapy prior to autologous stem cell transplantation in multiple myeloma. Leukemia. 2015; 29: 2429-2431.