Retrospective Study Comparing Effect of Sequential and Concurrent Hormonal Therapy with Radiation Therapy in Breast Cancer
Author'(s): Rasha Mohamed Abdel Latif*and Dalia Hatem Zayed
Clinical Oncology & Nuclear Medicine department, Faculty of medicine, Mansoura University, Egypt.
*Correspondence:
Rasha Mohamed Abdel Latif, Clinical Oncology & Nuclear Medicine department, Faculty of medicine, Mansoura University, Egypt.
Received: 14 May 2019; Accepted: 01 June 2019
Citation:Rasha Mohamed Abdel Latif, Dalia Hatem Zayed. Retrospective Study Comparing Effect of Sequential and Concurrent Hormonal Therapy with Radiation Therapy in Breast Cancer. Cancer Sci Res. 2019; 2(2); 1-7.
Abstract
Background: Radiation is a main component of treatment in patients with early breast cancer operated with conservative breast surgery (CBS), the sequence of adjuvant hormonal therapy with radiation when indicated was matter of question.
Objectives: In this study we tried to assess if using hormonal concurrently versus sequentially with radiation differs in relapse free and overall survival, in addition to the effect in developing toxicity (lung fibrosis, breast fibrosis and cardiac toxicity).
Patients and Methods: This is a retrospective study included 112 patients with early breast cancer treated with CBS and radiotherapy, patients divided into two groups according to sequence of hormonal treatment, 1st concurrent CON (received hormonal with radiation) and 2nd sequential SEQ (received hormonal after end of radiation. We compared outcomes of both groups.
Results: Concurrent group was 60 and SEQ was 52 patients, there was homogeneity in patientsËŠ characteristics between both groups, only the CON group received more taxane containing chemotherapy (P=0.04). There were no significant difference in ipsilateral relapse free (P=0.5), contralateral breast-relapse free (0.066), or overall survival (OS) (P=0.62). Distant metastasis free survival was higher in CON group (P=0.05). Lung fibrosis and breast fibrosis showed significant difference between CON and SEQ groups (P=0.004 and 0.018 respectively).
Conclusion: This study may suggest the use of hormonal therapy concurrently with radiation not inversely affect local or distant control, however this need more large numbers studies to improve this depate.
Keywords
Introduction
Breast cancer is the commonet cancer affecting females and represents 32.04% in Egyptian women [1]. Women with early stage carry good prognosis with 95% 5years survival [2]. Early stage breast cancer treated by radical mastectomy alternatively with conservative breast surgery (CBS) in conjunction with radiation therapy (RT), both have equivalent outcomes [3-5].
Breast cancer with positive estrogen (ER) or progesterone receptors (PR) treated with adjuvant Tamoxifen (TAM) in premenopausal and Aromatase Inhibitors (AI) in postmenopausal women is associated with increase in relapse-free survival and overall survival (OS) [6- 8]. Similarly, addition of adjuvant systemic therapy to CBS and RT affected both local control and distant relapse [4,9,10].
There was argument about timing of hormonal therapy with RT in treatment patients with CBS. This may be attributed to the superior results of disease free survival (DFS) and overall survival (OS) obtained when hormonal administered after chemotherapy over concurrently used with chemotherapy [11], this was explained by rendering cells less sensitive to cytotoxic effect of chemotherapy with using concurrent TAM [12,13].
There were studies which found that concurrently applying hormonal therapy with RT produce protective effect [14-16]. Although, of the previous results, recent studies have supposed a synergistic effect from TAM and letrozole in increasing apoptosis induced by radiation [17,18].
Both Tamoxifen (TAM) and Aromatase Inhibitors (AI) studied in this era and found comparable results of DFS and OS either given concurrently or sequentially with RT [18,19].
The studies compared sequential versus concurrent hormonal therapy with radiation evaluated toxicity like lung fibrosis and breast fibrosis, and they recorded increase in low grade breast [20] and lung fibrosis [21] in patients received concurrent arm.
In the present study we try to evaluate and compare relapse free survival, OS and toxicity between concurrent versus sequential hormonal with RT in patients operated with conservative breast surgery.
Patients and Methods
This is a retrospective study done in Clinical Oncology & Nuclear Medicine department in Mansoura University hospital between 2009-2013. Data were collected from files. Patients were divided into two groups, concurrent (CON), and sequential group (SEQ) according to time of administration of adjuvant hormonal treatment in relation to radiation treatment.
Study Objectives
The primary end point of this study to compare rates of local recurrence, contralateral breast cancer and distant metastasis between both groups. Secondary end point is evaluation of overall and relapse free survival of both groups, with assessment of toxicity (lung fibrosis, breast fibrosis and cardiac toxicity).
Inclusion criteria
The study included female patients above 18 years and ≤ 65 years, with early stage (stage I and II) unilateral breast cancer underwent conservative breast surgery (CBS) which performed by primary excisional biopsy with pathological negative margin, if excision associated with positive-margin re-excision was performed to clear margin. Patients received radiation therapy after CBS, and who received adjuvant hormonal therapy either Tamofen or Aromatase Inhibitors (AI). Patients received adjuvant chemotherapy were eligible.
Exclusion criteria
Patients with non-invasive carcinoma or advanced disease, previous or synchronous contralateral breast cancer, and or with other malignancies. Patients were excluded if data for sequencing hormonal with radiation therapy were unavailable. Associated lung and/or cardiac morbidity were excluded.
Treatment design
Patients were divided for comparisons of outcomes into 2 groups, sequential and concurrent. The sequential group (SEQ) defined as patients received adjuvant hormonal treatment after completion of radiation therapy. The concurrent group (CON) was defined as who received concurrent adjuvant hormonal with radiation, in which hormonal started before or with and continued with radiation therapy for any numbers of days.
Radiotherapy was delivered to whole breast to total dose of 50 Gy in 2-Gy fractions, followed by boost using electron beam to primary tumor bed to reach a total dose of 60 Gy. Radiation delivered to regional lymphatics when indicated as described elsewhere.
Systemic therapy in the form of chemotherapy and/or hormonal were used in accordance to standard community practice, as clinically indicated, and in keeping with patients and physician preference. Hormonal therapy was generally administered for a total of 5-years.
Outcomes
The outcomes for 2 groups included rates of ipsilateral recurrence, contralateral breast cancer, distant metastasis, and overall survival (OS). Local failure was documented by biopsy. Overall survival was calculated from date of pathological examination till death or lost follow up, local failure and distant metastasis were calculated from time of surgery till time of the specific event occur. Length of follow up was determined from end of radiotherapy till death or lost follow up. Toxicity in the form of lung fibrosis, breast fibrosis and cardiac toxicity also were recorded.
Follow up
Patients were followed after surgery, either in CON or SEQ group, clinically during radiotherapy for any acute toxicity and every follow up visit. Breast fibrosis was assessed post-radiotherapy with breast sonography and biopsy for confirmation. Lung fibrosis was evaluated with appearance of symptoms by chest x-ray or chest computed tomography (CT), which available. Finally, cardiac toxicity assessed when patient complain with ECHO for any changes detected in relation to ECHO performed before chemotherapy administration.
Statistical analysis
Data was analyzed using Statistical Package for Social Science software computer program version 23 (SPSS, Inc., Chicago, IL, USA). Quantitative parametric data were presented in mean and standard deviation, while Qualitative data were presented in frequency (Number-percent). Student's t-test (unpaired) was used for comparing two groups with quantitative parametric. Chi- square “χ2” was used to compare the qualitative data. Kaplan– Meier curves were generated to compare disease free survival & overall survival of patient’s method of treatment. Univariate and multivariate Cox proportional hazards regression models were used to calculate the hazard ratios of disease free survival & overall survival to detect predictors. P value less than 0.05 was considered statistically significant.
Results
This is a retrospective study included 112 cases attended to Clinical Oncology& Nuclear Medicine department, Mansoura University Hospital in the period between January 2009 to December 2013, with early stage breast cancer (stage I and II) operated with conservative breast surgery (CBS).
PatientsË? characteristics of the two groups are listed in table (1), 60 patients were in the concurrent (CON) group and 52 patients in the sequential one (SEQ). No significant difference detected between 2 groups in clinic-pathological characters apart from type of chemotherapy and time of follow up. The SEQ group received Anthracyclin containing chemotherapy more than the CON group who received Taxane-containing chemotherapy more than SEQ (P=0.04). The mean of follow up of CON (82.7 SD ± 21.5) was longer than that of SEQ (76.5 SD ± 26.7) (P Ë? 0.001). developed contralateral breast cancer in SEQ group (P=0.06). Distant metastasis were diagnosed in 16 patients in SEQ group which was more than in CON group (10 patients only), but was not significant statistically (P=0.08).
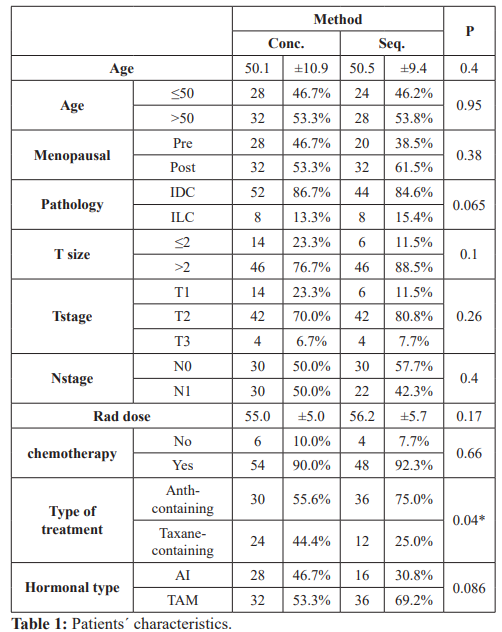
CON: Concurrent, SEQ: Sequential, Pre: Premenopausal, Post: Postmenopausal, IDC: Infilterating duct carcinoma, ILC: Infilterating lobular carcinoma, T: Tumor, N: Lymph node, rad.dose: Radiation dose, Anth: Anthracyclin, AI: Aromatase inhibitors, TAM: Tamoxifen.
Table 2, shows the outcomes of the patients. Overall deaths were reported in 10 patients in each group (P=0.8). Ipsilateral recurrence was equal in both groups in 8 patients (P=0.75). Four patients in the CON group had contralateral breast cancer, but no patient
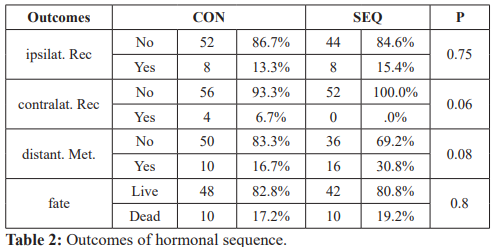
Ipsilat Rec: Ipsilateral recurrence, Contralat. Rec: Contralateral recurrence, distant. Met.: Distant metastasis.
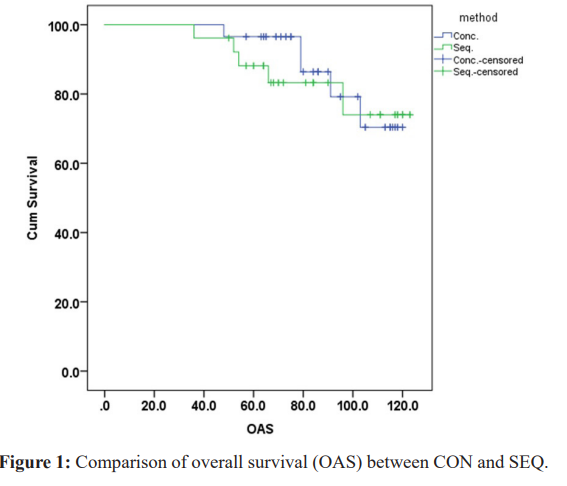
The overall survival (OAS) of the 2 groups was almost the same, the mean OAS of CON group was 109.767months (95% CI 104.145-115.389), and 108.756 months (95%CI 100.874-116.639) in SEQ group, (P=0.62), (Figure 1).
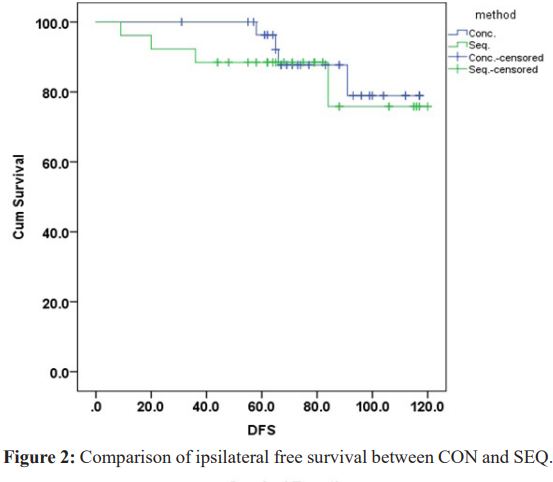
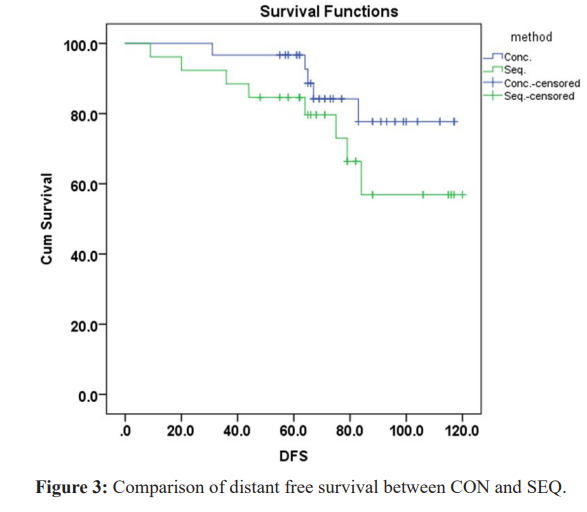
The ipsilateral recurrence free survival was slightly higher in CON group 108.120 months (95%CI 102.529-113.710), while in SEQ group was 104.104 months (95%CI 94.074-114.135), but not significant (P=0.5) (Figure 2). Although, no patients developed contralateral breast cancer in SEQ group, the contralateral breast cancer free survival in the CON group did not reach statistical significance (P=0.066). The distant metastasis free survival was significantly higher in CON versus SEQ {mean 105.487 months (95%CI99.023-111.951) vs 93.823 months (95%CI 83.336-104.310), respectively}, P= (0.05) (Figure 3).
On univariate analysis for factors affecting ipsilateral recurrence, the T3 stage tumor exhibited significant difference versus TI and T2 stages (HR 7.985, 95%CI 2.370-26.907, P=0.001), CON vs SEQ showed no significant statistical difference (HR, 1.394, 95%CI 0.521-3.726, P=0.508), also other adjusted factors namely the age, menopausal status, pathology, N stage, chemotherapy, and hormonal type were associated with no significant difference. On multivariate analysis using cox regression, the T stage showed significant difference of T3 vs T1+2 (HR, 10.460, 95% CI 2.886- 37.917, P=0.001).
Regarding contralateral recurrence, only univariate analysis performed as no cases reported contralateral recurrence in sequential group, and no factors presented significance.
Univariate and multivariate analysis performed for distant metastasis, N stage showed significant difference on univariate analysis (HR 2.710, 95% CI 1.177-6.243, P= 0.019), but no significance on multivariate (HR 2.223, 95% CI 0.943-5.241, P= 0.068).
When factors adjusted for OAS, on univariate analysis, factors associated with significant impact were, age (HR 4.511, 95% CI 1.500-13.563, P= 0.007), menopausal status (HR 3.833, 95% CI 1.276-11.520, P= 0.017), ipsilateral recurrence (HR 3.184, 95% CI 1.221-8.303, P= 0.018), and distant relapse (HR 13.059, 95% CI 4.359-39.129, P= Ë?0.001). However, CON vs SEQ was not associated with significant impact (HR 1.242, 95% CI 0.516- 2.987, P = 0.629) with other factors too. On multivariate, only distant metastasis showed statistical significance (HR 13.902, 95%CI 4.604-41.972, PË? 0.001), (Table 3).
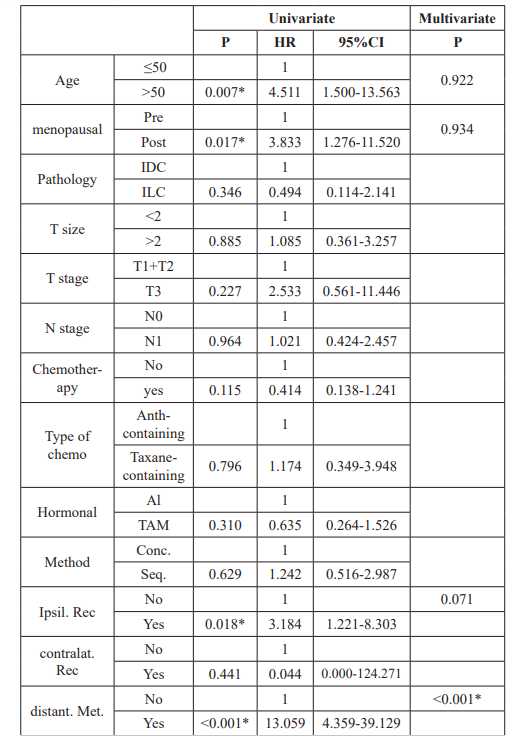
Table 3: Univariate and multivariate analysis of factors affecting OS. Pre: Premenopausal, Post: Postmenopausal, IDC: Infilterating duct carcinoma, ILC: Infilterating lobular carcinoma, T: Tumor, N: Lymph node, Anth: Anthracyclin, AI: Aromatase inhibitors, TAM: Tamoxifen. CON: Concurrent, SEQ: Sequential, Ipsilat Rec: Ipsilateral recurrence, Contralat. Rec: Contralateral recurrence, distant. Met.: Distant metastasis.
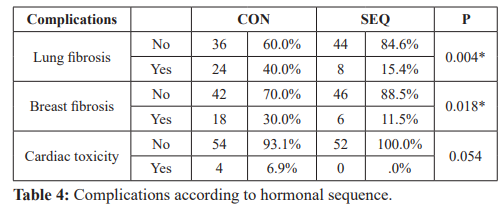
Complications compared between CON and SEQ groups (Table 4). Complications assessed were lung fibrosis, breast fibrosis, and cardiac toxicity. Twenty four patients developed lung fibrosis in CON vs SEQ as only 8 patients had lung fibrosis with significant difference (P= 0.004). Six patients only developed breast fibrosis in SEQ group while, 18 suffered in CON group and was statistically significant (P= 0.018). No patients had cardiac toxicity in SEQ group (P= 0.054).
Discussion
Women with early breast cancer worldwide are treated with breast- conserving surgery followed by radiation, adjuvant chemotherapy, and/or hormonal therapy and this is based on data obtained from many randomized trials. However, despite the extensive use of combinations of the three lines, the best sequencing of these different lines for those patients remains unknown yet .There have been few randomized studies dealing with this crucial issue.
Radiation therapy and adjuvant chemotherapy are given sequentially. Many chemotherapeutics agents such as doxorubicin and methotrexate are considered as radio-sensitizers, and concurrent administration of these drugs are associated with an increased risk of radiation side effects affecting skin, soft tissue and heart [19]. Moreover, Tamoxifen used concurrently with chemotherapy increased risk of thromboembolic event [22].
The best sequence for adjuvant endocrine and RT represented a challenge for the oncologists and a matter of a depate. Tamoxifen is a cytostatic drug that arrests cells in G1, a relatively radio- resistant cell cycle phase [23]. As endocrine therapy has no significant radio-sensitizing properties; therefore, it has been given concurrently with radiation [19]. Therefor patients receiving concurrent Tamoxifen and radiation remains at an increased risk of adverse effects [24].
For the Aromatase Inhibitor treatment, In vitro results by Azria et al. [25] showed that letrozole sensitizes breast cancer cells to radiation in the range of 0 to 4 Gy. These results supporting concurrent use of Aromatase Inhibitor and RT in postsurgical settings for more clinical efficacy. However, this synergistic effect might theoretically translate into more side effects, most data from literature suggest safety of delivering AI concurrently with adjuvant RT.
The FEMTABIG study (letrozole vs. tamoxifen vs. sequential treatment), and the ATAC study compared anastrazole (Aramidex) and tamoxifen did not specify the sequence of radiation and hormonal therapy. The TEAM study compared exemestane (Aromasine) and tamoxifen, but specified that hormonal treatment follow the completion of radiation therapy [26].
This is a retrospective study done on a 112 patients ,that assess the effect of the sequence of hormonal therapy (Tamofen or Aromatase Inhibitors) with RT on outcomes in early breast cancer patients (stage I and II), who underwent breast-conservative surgery , with or without adjuvant chemotherapy. Patients were grouped as concurrent (hormonal therapy given before or during RT followed by continued hormonal treatment for 5 years (60 patients) and sequential (RT followed by hormonal therapy for 5 years also (52 patients).
This current study showed homogeneity regarding the patient’s clinico-pathological features with the exception of the type of the chemotherapy taken, that depended mainly on the decision of the treating physician. We noticed that the concurrent group of patients who had a statistically significant longer period of follow up, received more (Taxene- containing chemotherapy) which was a relatively newer agent at that time and there was a gradual shift in our clinical practice to use RT and hormonal treatment concurrently due to many studies released during this period.
Our study showed no significant statistical difference between the two groups in terms of the overall survival .This is similar to that obtained from a number of retrospective studies [19,27-30] and to be more specific, the data for Tamoxifen mainly depends on three retrospective studies with a total number of 1,082 patients and a period of follow-up ranged between eight to 10 years for the individual studies [19,27,30] which is comparable to our own data.
The distant metastasis free survival was significantly higher in the concurrent group versus the sequential one P= (0.05), which could be attributed to the relatively more usage of novel chemotherapy agents (Taxene-containing regimens) at that time in the first group unlike the sequential group that used more of the (Anthracyclin– containing regimens). However the difference in the number of patients that developed metastases in both groups did not reach a statistical significance and this is maybe due to the relative small size group of patients.
Multiple known factors influence the severity of acute and late reactions following RT, for example the total dose, observation time fractionation, beam energy, or type of surgery. Our study population was quite homogenous regarding these factors.
In this study, complications assessed following adjuvant treatment were lung fibrosis, breast fibrosis, and cardiac toxicity. Twenty four patients developed lung fibrosis (as evidenced by chest CT) in CON vs SEQ as only 8 patients had lung fibrosis with significant difference (P= 0.004). Four patients only in the concurrent group had clinical symptoms of radiation pneumonitis compared to three patients in the other group.
There are little data on the risk of complications with concurrent use. One old study reported an increased risk of lung fibrosis in post mastectomy patients received radiotherapy and Tamoxifen [31].
However, in another study, used chest computed tomography scans to quantify radiographic changes in the lungs (were performed before radiation and 4 months after completion radiation therapy) and correlate these with radiation technique and symptomatic pneumonitis [32]. No correlation between lung density changes found radiologically and the use of concurrent use of Tamoxifen and radiotherapy.
In our study, the incidence of breast fibroses was statistically significant higher in the concurrent group with a P value of 0.018. There is evidence of increased rates of low-grade breast fibrosis [20,33] and lung fibrosis [31,34] in patients treated with concurrent Tamoxifen compared to patients treated with radiation alone.
On the other hand another large retrospective study (278 patients) with a median follow up of 8.6 years identified no difference in the rates of breast edema, breast fibrosis or arm edema, between the two groups [19]. More interestingly another retrospective study that assessed the acute and long-term toxicity using aromatase inhibitors (AI) therapy concurrently with hypo-fractionated radiotherapy (HFRT) on 66 breast cancer patients. All toxicities were mild to moderate, and no treatment disruption was necessary. Further prospective assessment is warranted [35].
Toxicity to the heart has been identified as an important adverse event of radiation to the chest [36]. Although, the majority of radiation for breast is delivered as photon tangents with posterior divergence, still an appreciable dose is delivered to the heart, especially in left-sided breast cancer. In the current study, cardiac toxicities were reported in the concurrent group (6.9% vs 0%) but with no statistical significance (P=0.054). To date, this important question has not been addressed in clinical studies. As, younger premenopausal women receive Tamoxifen, are likely to have significant life spans after treatment, in some cases, 30 years or more, if there are increased rates of cardiac fibrosis, this may put them at higher risks of late cardiac events and warrants further study.
As the sequential sequence has not been associated with any harm in clinical outcomes, and there is some suggestions of increased toxicity with concurrent use as shown in this study and some others. Further prospective randomized studies have to be done on large number of patients with a homogenous clinic-pathological features, especially with a sufficient periods of follow up for better evaluation of cancer outcomes and late toxicities .In addition to that, for patients at high risk of distant recurrence, it would be reasonable to consider concurrent treatment to avoid any delay in therapy.
References
- Amal S, Hussein M, Nabiel NH, et al. Cancer Incidence in Egypt Results of the National Population-Based Cancer Registry Journal of Cancer Epidemiology. 2014; 18.
- Newman Epidemiology of locally advanced breast cancer. Semin Radiat Oncol. 2009; 19: 195-203.
- Fisher B, Anderson S, Redmond CK, et Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995; 333: 1456-1461.
- Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma Long- term results of a randomized trial. Ann Oncol. 2001; 12: 997-
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival an overview of the randomised Early Breast Cancer Trialists' Collaborative Group EBCTCG. Lancet. 2005; 366: 2087-2106.
- Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of Aromatase Inhibitors versus J Clin Oncol. 2010; 28: 509-518.
- Davies C, Godwin J, Gray R, et Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant Tamoxifen patient-level meta-analysis of randomized trials. Early Breast Cancer. 2011; 378: 771-784.
- Early Breast Cancer Trialists Collaborative Tamoxifen for early breast cancer An overview of the randomised trials. Lancet. 1998; 351: 1451-1467.
- Haffty BG, Fischer D, Rose M, et al. Prognostic factors for local recurrence in the conservatively treated breast cancer patient A cautious interpretation of the data. J Clin Oncol. 1991; 9: 997-1003.
- Anglian Breast Cancer Study Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population- based series of breast cancer cases: Br J Cancer. 2000; 83: 1301-8130.
- Albain KS, Green SJ, Ravdin PM, et al. Adjuvant chemo- hormonal therapy for primary breast cancer should be given sequential instead of concurrent Initial results from Intergroup trial 0100 SWOG-88-14. J Clin 2002; 21: 37.
- Hug V, Hortobagyi GN, Drewinko B, et Tamoxifen-citrate counteracts the antitumor effects of cytotoxic drugs in vitro. J Clin Oncol. 1985; 3: 1672-1677.
- Jakesz R, Hausmaninger H, Haider K, et Randomized trial of low-dose chemotherapy added to Tamoxifen in patients with receptor-positive and lymph node-positive breast cancer. J Clin Oncol. 1999; 17: 1701-1709.
- Wazer DE, Tercilla OF, Lin PS, et al. Modulation in the radiosensitivity of MCF-7 human breast carcinoma cells by 17B-estradiol and Tamoxifen. Br J Radiol. 1989; 62: 1079-
- Paulsen GH, Strickert T, Marthinsen AB, et al. Changes in radiation sensitivity and steroid receptor content induced by hormonal agents and ionizing radiation in breast cancer cells in Acta Oncol. 1996; 35: 1011-1019.
- Villalobos M, Aranda M, Nunez MI, et Interaction between ionizing radiation estrogens and antiestrogens in the modification of tumor microenvironment in estrogen dependent multicellular spheroids. Acta Oncol. 1995; 34: 413-417.
- Zeng ZJ, Li JH, Zhang YJ, et Optimal combination of radiotherapy and endocrine drugs in breast cancer treatment. Cancer Radiother. 2013; 17: 208-214.
- Azria D, Larbouret C, Cunat S, et al. Letrozole sensitizes breast cancer cells to ionizing radiation. Breast Cancer Res. 2005; 7: 156-163.
- Harris E, Christensen V, Hwang W, et Impact of Concurrent Versus Sequential Tamoxifen with Radiation Therapy in Early- Stage Breast Cancer Patients Undergoing Breast Conservation Treatment. J Clin Oncol. 2005; 23: 11-16.
- Azria D, Gourgou S, Sozzi WJ, et al. Concomitant use of Tamoxifen with radiotherapy enhances subcutaneous breast fibrosis in hypersensitive patients. Br J Cancer. 2004; 91: 1251-1260.
- Koc M, Polat P, Suma Effects of Tamoxifen on pulmonary fibrosis after cobalt-60 radiotherapy in breast cancer patients. Radiother Oncol. 2002; 64: 171-175.
- Pritchard K, Paterson H, Paul N, et Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J Clin Oncol. 1996; 14: 2731-2737.
- Chargari C, Toillon RA, Macdermed D, et al. Concurrent hormone and radiation therapy in patients with breast cancer what is the Lancet Oncol. 2009; 10: 53-60.
- Osborne C, Boldt D, Clark G, et al. Effects of tamoxifen on human breast cancer cell cycle kinetics Accumulation of cells in early G1 Cancer Res. 1983; 43: 3583-3585.
- Azria D, Belkacemi Y, Romieu G, et Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer CO-HO-RT a phase 2 randomised trial. The Lancet Oncology. 2010; 11: 256-265.
- Azria D, Lemanski C, Zouhair A, et Adjuvant treatment of breast cancer by concomitant hormonotherapy and radiotherapy state of the art. Cancer Radiothérapie. 2004; 8:188-196.
- Pierce LJ, Hutchins LF, Green SR, et Sequencing of tamoxifen and radiotherapy after breast-conserving surgery in early-stage breast cancer. J Clin Oncol. 2005; 23: 24-29.
- Ishitobi M, Shiba M, Nakayama T, et al. Treatment sequence of aromatase inhibitors and radiotherapy and long-term outcomes of breast cancer Anticancer Res. 2014; 34: 4311-4314.
- Valakh V, Trombetta MG, Werts ED, et Influence of concurrent anastrozole on acute and late side effects of whole breast radiotherapy. Am J Clin Oncol. 2011; 34: 245-248.
- Ahn PH, Vu HT, Lannin D, et al. Sequence of radiotherapy with tamoxifen in conservatively managed breast cancer does not affect local relapse J Clin Oncol. 2005; 23: 17-23.
- Bentzen S, Skoczylas J, Overgaard M. Radiotherapy-related lung fibrosis enhanced by J Natl Cancer Inst. 1996; 88: 918-922.
- Wennberg B, Gagliardi G, Sundbom L, et al. Early response of lung in breast cancer irradiation Radiologic density changes measured by CT and symptomatic radiation Int J Radiat Oncol Biol Phys. 2002; 52: 1196-1206.
- Johansen J, Overgaard J, Overgaard M. Effect of adjuvant systemic treatment on cosmetic outcome and late normal tissue reactions after breast conservation. Acta Oncol. 2007; 46: 525-533.
- Varga Z, Cserháti A, Kelemen G, et al. Role of systemic therapy in the development of lung sequelae after conformal radiotherapy in breast cancer Int J Radiat Oncol Biol Phys. 2011; 80: 1109-1116.
- Chargari C, Castro-Pena P, Toledano I, et Concurrent use of aromatase inhibitors and hypofractionated radiation therapy. World J Radiol. 2012; 4: 318-323.
- Darby SC, Ewertz M, McGale P, et Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013; 368: 987-998.