Risk Factors for Hepatic Fibrosis in Patients with Chronic Viral Hepatitis B at the Hospital National Donka CHU in Conakry
Author'(s): Diallo Mamadou Sarifou1,2*, Youssouf Oumarou3, Diallo Ahmed Tidiane1,2, Diallo Kadiatou1,2, Diallo Djenabou1,2, Wann Thierno Amadou5,2, Bah Mamadou LamineYaya5,2, Diakhaby Mamadou5, Kanté Mamadou Aliou5, Sylla Djibril5,2, Soro Dramane4 and Diallo Abdourahmane N'Djouria6
1Hepato-Gastroenterology Department of the Donka National Hospital, Conakry, Guinea.
2Faculty of Health Sciences and Techniques, Gamal Abdel Nasser University, Conakry, Guinea.
3Centre Hospitalier Universitaire Communautaire de Bangui, Internal Medicine Department, Central African Republic.
4Hepato Gastroenterology Department, Cocody University Hospital, Abidjan, Ivory Coast.
5Department of Internal Medicine, Donka National Hospital, Conakry, Guinea.
6SOS Hepatites Guinea.
*Correspondence:
Dr. Mamadou Sarifou DIALLO, Hepato-Gastroenterology Department Donka National Hospital CHU Conakry, Guinea, Tel: 00224628690551.
Received: 05 Apr 2024 Accepted: 08 May 2024 Published: 16 May 2024
Citation: Diallo M Sarifou, Youssouf Oumarou, Diallo A Tidiane, et al. Risk Factors for Hepatic Fibrosis in Patients with Chronic Viral Hepatitis B at the Hospital National Donka CHU in Conakry. Gastroint Hepatol Dig Dis. 2024; 7(2): 1-6.
Abstract
Introduction: Viral hepatitis B is a worldwide public health problem. Hepatic fibrosis is the consequence of a prolonged fibrinogenesis mechanism. It is caused by all chronic liver diseases, essentially of viral origin. The progression of fibrosis leads to cirrhosis and its complications. Fibroscan®, or elastometry, is a non-invasive approach capable of assessing liver fibrosis, particularly in the management and monitoring of patients with chronic liver diseases, such as hepatitis B and C or cirrhosis.
Methods: This was a prospective observational study with descriptive and analytical aims. It was carried out from 1er October 2022 to 31 July 2023, i.e. 10 months, on an outpatient basis in the hepato-gastroenterology department of the Donka National Hospital of the University Hospital of Conakry.
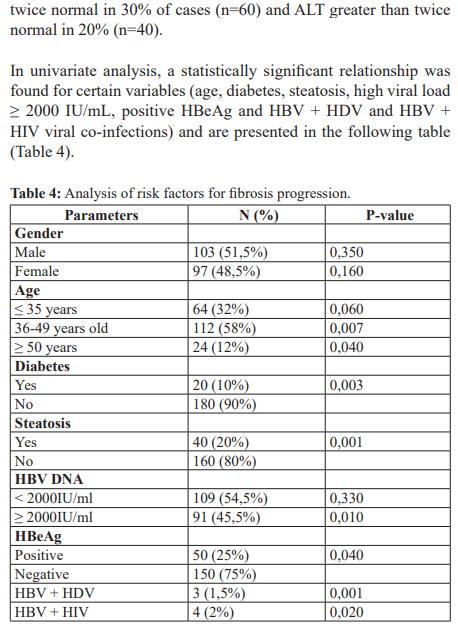
Results: The main limitations of this study were the small sample size, the high cost of pulse elastometry, and the absence of antigenemia (quantitative HBsAg). Of the 200 patients included, 103 were men (51.5%) and 97 women (48.5%), giving a sex ratio of 1.06. The mean age was 36 years, with extremes of 18 and 77 years. The 36-49 age group was the most affected, with a frequency of 58% (n=112). Hepatic fibrosis on Fibroscan was distributed as follows: 51% (n=102) had no fibrosis or minimal fibrosis (F0F1), 30% (n=60) with moderate fibrosis (F2), 17.5% (n=35) with severe fibrosis (F3) and 2.5% (n=5) with fibrosis classified as F4. Hepatic steatosis 51% (n=102) of our patients were at stage S0, 29% (n=58) at stage S1, 12.5% (n=25) at stage S2 and 7.5% (n=15) at stage S3. In univariate analysis, the main risk factors for progression of liver fibrosis with a statistically significant association in our series were: age (p= 0.007), diabetes (p= 0.003), steatosis (p=0.001), high HBV DNA greater than or equal to 2000UI/mL (p= 0.002), HBeAg positivity (p=0.04), co-infections with hepatitis D and HIV viruses with a p-value of 0.001 and 0.020 respectively.
Conclusion: Appropriate management of chronic HBV carriage can limit the risk of progression to cirrhosis and HCC, thereby reducing morbidity and mortality. The asymptomatic nature of the infection is a factor in the spread of the epidemic, and may be responsible for late diagnosis at an advanced stage of the disease. Policies must encourage widespread screening and universal vaccination against the hepatitis B virus as a matter of course, in order to reduce the risk of new infections and hence of HBV-related complications. Knowledge and identification of the risk factors for the progression of hepatic fibrosis is essential if complications are to be prevented.
Keywords
Introduction
Infection with the hepatitis B virus (HBV) is associated with high morbidity and mortality, particularly due to the risk of progression to cirrhosis and hepatocellular carcinoma (HCC). This unfavourable progression depends on numerous factors linked to the virus, the host and the environment. Knowledge and identification of these risk factors is essential if these complications are to be prevented [1]. Hepatitis B is a global public health problem, with an estimated 316 million chronic HBV carriers in 2019 and 820,000 HBV-related deaths in 2015, mainly due to the development of cirrhosis or HCC [2]. Hepatitis B is distributed worldwide, with highly variable prevalence from one region to another. Sub-Saharan Africa is a highly endemic region, with 65 million chronic carriers and 56,000 deaths per year. In these regions, HBV is mainly transmitted vertically from mother to child, or horizontally during the perinatal period through contact with an infected person. The populations at risk are therefore children born to mothers with hepatitis B and contact subjects [3,4]. In Guinea Conakry, no nationwide study has been carried out to determine the national seroprevalence of viral hepatitis. However, a few piecemeal studies have produced significant results. For example, between 1998 and 2001, the seroprevalence of HBsAg carriage among blood donors at the national blood transfusion centre was 14.85% [5]. In 2003, in the endocrinology department of the Conakry University Hospital, seroprevalence was 8.06% among diabetic patients admitted to hospital [6]. Hepatic fibrosis is the main complication of chronic liver disease. Progression of fibrosis eventually leads to cirrhosis, a source of high morbidity and mortality. The quantification of fibrosis is important, as it conditions both prognosis and therapeutic indications [7]. The assessment of fibrosis is an important element in the management of chronic liver disease [8]. Liver biopsy (LBB) is the gold standard for investigating liver pathology, but its morbidity and mortality and its cost limit its use to specific indications [9]. Fibroscan, on the other hand, is a non-invasive examination based on exploration of the liver using pulse elastometry, and is well suited to chronic viral hepatitis B and C as well as steatosis. Fibroscan is an innovative technology for measuring liver hardness non-invasively, painlessly and immediately [10,11]. In Guinea Conakry, to our knowledge, no study has been carried out on this subject. Given this situation, we undertook this study with the aim of identifying the risk factors associated with the progression of liver fibrosis in patients with chronic HBV followed in our department.
Materials and Methods
We conducted a prospective observational study with descriptive and analytical aims. It was carried out from 1er October 2022 to 31 July 2023, i.e. 10 months, on an outpatient basis in the hepato- gastroenterology department of the Donka National Hospital of the University Hospital of Conakry.
The study included all patients of any age, sex and origin with chronic viral hepatitis B who had undergone FIBROSCAN during the study period and who had agreed to take part in the study by giving oral consent.
The criteria for non-inclusion were:
− Patients with incomplete HBV serology.
− Patients who did not undergo FIBROSCAN.
− Patients who refused to take part in the study.
− All decompensated cirrhotic patients.
Sociodemographic, clinical and ultrasound parameters:
− Age: 18 to 77
− Sex
− Personal history: diabetes, arterial hypertension (hypertension in mmHg), dyslipidaemia, etc.
Liver fibrosis was graded using the result in kilo Pascal (kPa):
− No fibrosis or minimal fibrosis = F0 F1, when liver elasticity
is less than 2.5 to 7.5 kPa
− Presence of F2 fibrosis when liver elasticity is between 7.5 and 9.5 kPa
− Presence of severe fibrosis F3, when liver elasticity is between 9.5 and 12.5 kPa
− Presence of F4 cirrhosis, when liver elasticity is greater than 12.5 kPa
Fibroscan values ≥ F2 are considered significant (moderate to severe lesions). Measurement of hepatic steatosis by CAP with Fibroscan:
− S0 steatosis or absence of steatosis if CAP for lower values between 100 -238 dB/m (0-10%)
− Stage I or S 1 steatosis for values between 238 and 260 dB/m (11-33%)
− Stage S 2 steatosis for values between 260 and 290 dB/m (34- 66%)
− Stage S 3 steatosis for values above 290 - 400 dB/m (> 67%) We considered stages S2 and S3 to be significant steatosis requiring follow-up.
The fibroscan/ CAP enables both elasticity correlated with hepatic fibrosis and CAP correlated with steatosis to be measured. Results are expressed in kPa for elasticity and correspond to the median of 1 to valid measurements. The CAP measurement is guided by the elasticity measurement, is expressed in dB/m and corresponds to ultrasound attenuation. It describes the decay of the ultrasound signal as a function of depth. The greater the amount of steatosis in the liver, the greater the decay, and it is only calculated if the elasticity measurement is valid.
In order to interpret the results, the following two factors must be taken into account: the variability of valid measurements, assessed by the value of the interquartile range (IQR) displayed by the machine, which must be less than 30% of the median, and the success rate (number of measurements compared with the number of measurements taken), which must be greater than 60% to be considered satisfactory.
The fibroscan was performed in patients who had been fasting for 3 hours prior to the examination. Patients were positioned supine, with their torso undressed, and their right arm folded under the head in extension to clear the intercostal spaces. The probe was placed perpendicular to the skin between the 9ème and 11ème right intercostal space on the mid-clavicular line and the measurements were acquired.
The M probe was used for patients with a BMI of less than 30 kg/m2 and the XL probe for patients with a BMI ≥ 30 kg/m2 or in patients where we had difficulty obtaining valid measurements with the M probe; we did not have the S probe on our tray.
The examination was carried out using the Fibroscan 502 Touch (SN F60782) Echosens, France. All examinations were carried out by a senior staff member and by the same doctor. The biological parameters studied were: fasting glycaemia, transaminase levels: Alanine aminotransferase (ALAT in IU/l) and aspartate aminotransferase (ASAT in IU/l), HDL cholesterol in g/l, LDL cholesterol, total cholesterol, TG triglyceride levels, albumin (g/l), creatinine, phosphoremia, alpha feto protein. Virological parameters: HBsAg, total HBcAg, HBeAg, HBeAg, HBV DNA, HCVAg, HCV RNA, HDVAg, HDV RNA, quantitative HBsAg (antigenemia) not available, retroviral serology (SRV) for acquired immunodeficiency virus (HIV).
Abdominal ultrasound to study the morphology of the liver and look for hepatic steatosis, defined as an increase in the echogenicity of the liver parenchyma compared with that of the right renal cortex. chronic HBsAg carriage is defined as persistence of HBsAg for more than 6 months.data were collected using an individual survey form developed for this purpose. In this study we respected ethical considerations, in particular: the moral and physical integrity of the person, the free and voluntary consent of the person, the confidentiality of the results and the anonymity of the persons interviewed, and the possible wish of the person interviewed to withdraw without prejudice. The hospital has consented to the use of the data of patients who have been consulted in the department.
Data were entered and analysed in EXCEL, ACCES 13 and SPSS18. Variable comparisons were made using Pearson's Chi- square test2 and Fisher's exact test. The significance threshold was 5%.
Results
Of the 200 patients included, 103 were male (51.5%) and 97 female (48.5%), giving a sex ratio of 1.06. The average age of our patients was 36, with extremes of 18 and 77. The 36-49 age group was the most affected with a frequency of 58% (n=112) followed by the ≤ 35 age group with a frequency of 32% (n=64) and the 50 and over age group with a frequency of 12% (n=24).
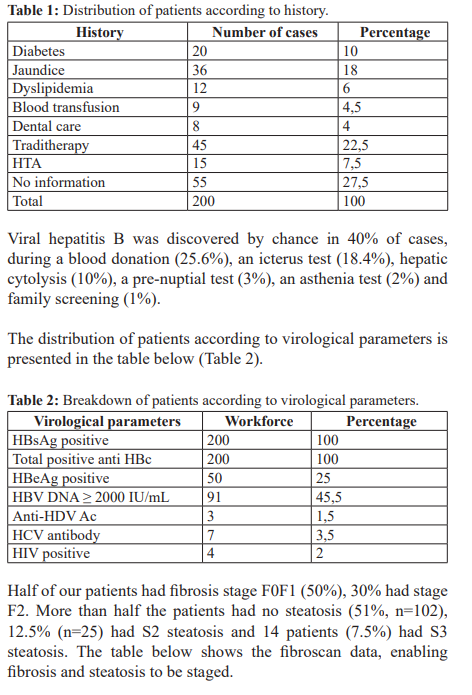
Our patients' histories are presented in the table below (Table 1).
Discussion
The main limitations of this study included the small size of our sample, the high cost of pulse elastometry (Fibroscan), the monocentric nature of the study, and the absence of antigenemia (quantitative HBsAg). Nonetheless, the study identified the main risk factors for the progression of liver fibrosis in patients with chronic hepatitis B virus.
M.S et al. [15] in Guinea found an average age of 40, with extremes of 18 and 88. This young average age could be explained by the predominance of vertical contamination from mother to child and horizontal contamination during infancy, which are the main modes of transmission in Guinea [4,5].
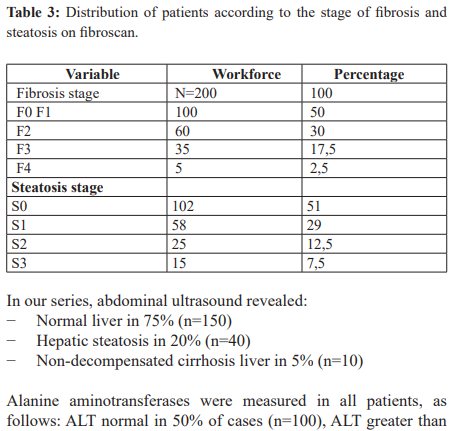
In our study, hepatic fibrosis on Fibroscan was distributed as follows: 51% (n=102) had no fibrosis or minimal fibrosis (F0F1), 30% (n=60) with moderate fibrosis (F2), 17.5% (n=35) with severe fibrosis (F3) and 2.5% (n=5) with fibrosis classified as F4 (Table 3). Our results are slightly lower than those reported by Seto et al. [16] who noted a prevalence of F3 and F4 fibrosis of 27.1% and 11.2% respectively in their population.
In their study, Diallo MS et al. reported hepatic fibrosis with the following frequencies: 58.82% (n=100) had no fibrosis or minimal fibrosis (F0F1), 22.94% (n=39) moderate fibrosis (F2), 11.76% (n=20) severe fibrosis (F3) and 6.47% (n=11) fibrosis classified as F4 [15].
Our results of F3 (17.5%) and F4 (2.5%) fibrosis are similar to those reported by Choi et al. in a study comparing two populations of chronic hepatitis B virus (HBV) carriers with and without non-alcoholic steatohepatitis diagnosed by biopsy, who found a higher prevalence of F3 and F4 fibrosis in patients with NASH (Non Alcoholic Steatohepatitis); respectively 14.7% and 9.9% in patients without NASH and 21.6% and 17.8% in those with NASH [17].
This higher prevalence of fibrosis in patients with HBV-NOSH could explain the hypothesis that the coexistence of these two pathologies increases the risk of these patients progressing to cirrhosis and hepatocellular carcinoma [18].
In our series, concerning hepatic steatosis, 51% (n=102) of our patients were at stage S0, 29% (n=58) at stage S1, 12.5% (n=25) at stage S2 and 7.5% (n=15) at stage S3 (Table 3). Our results are similar to those reported by Wang et al. [19] who found that 36.6% of patients assessed by liver biopsy were stage S0, 36.36% stage S1, 19.05% stage S2 and 9.09% stage S3. As for Diallo MS Viral hepatitis B affects both men and women, with a male predominance of 51.5% (n=103) compared with 48.5% in women, with a M/F sex ratio of 1.06. According to the literature, the predominance of males has been reported in several studies [9,12,13]. This male predominance could be explained by the lifestyle of men, who are more exposed than women to risk factors for viral infection: alcohol, polygamy, high-risk sexual behaviour.
The mean age of our patients was 36 years, with extremes of 18 and 77 years. The 36-49 age group was the most affected with a frequency of 58% (n=112) followed by the ≤ 35 age group with a frequency of 32% (n=64) and the 50 and over age group with a frequency of 12% (n=24).
Our results are similar to those of Mongo O et al. in Brazzaville [14], who reported an average age of 34 years. Similarly, Diallo et al. [15] in Guinea, 62 (36.47%) of their patients were at stage S0, 29.41% at stage S1, 20% at stage S2 and 14.11% at stage S3 (hepatic steatosis in 34.11%; n = 58).
In our study, we found no relationship between gender and low load (< 2000IU/mL) (Table 4). Several risk factors for the progression of liver fibrosis have been described in the literature (virological factors, host factors, environmental factors) and appear to be associated with the development of B cirrhosis. The risk factors best established in the literature are male sex, advanced age and a viral load greater than 2000IU/mL [20-22]. This difference could be explained on the one hand by vertical contamination from mother to child and horizontal contamination during infancy, which are the main modes of transmission in Guinea, and on the other hand by the young age of our patients, who therefore have fewer cardiovascular comorbidities.
In univariate analysis, the main risk factors for progression of hepatic fibrosis with a statistically significant association in our series were : age (p= 0.007), diabetes (p= 0.003), steatosis (p=0.001), elevated HBV DNA greater than or equal to 2000UI/ mL (p= 0.002), HBeAg positivity (p=0.04), co-infections with hepatitis D and HIV viruses with a p-value of 0.001 and 0.020 respectively (Table 4).
Our results are similar to those reported by Mallem M et al. in Algeria [22] who found the following to be the main risk factors for progression of hepatic fibrosis in patients with significant chronic viral hepatitis: diabetes (p<0.001), HBV DNA >2000IU/mL (p<0.001), male sex (p<0.001) and age over 40 years (p<0.001). However, there was no significant relationship between steatosis and HBeAg-positive status.
The progression of liver fibrosis in an individual is difficult to assess with certainty. It is a chronic, progressive process, with progression to cirrhosis occurring within 15-20 years. The severity of inflammation and liver damage is usually correlated with the rate of fibrosis progression. In alcoholic liver disease, the predominant factor in the progression of liver fibrosis is continued alcohol consumption. Other risk factors include hyperglycaemia [23].
In the course of viral liver disease, the risk factors for progression of hepatic fibrosis are as follows: high age at infection, concomitant excessive alcohol consumption, viral co-infection with D, C and HIV, male sex, increased body mass index (BMI) associated with steatosis and iron overload [23,24]. The role of diabetes in the progression of hepatic fibrosis during HBV infection remains controversial; however, a 2014 Chinese study states that diabetes increases the risk of developing hepatic fibrosis by a factor of 1.1, and another Asian study shows that there is a significant 5-fold increased risk of developing cirrhosis in diabetic patients infected with HBV, compared with non-diabetic infected patients [25,26].
Similarly, the relative risk of cirrhosis is proportional to the viral load. It is 2.5, 5.6 and 6.5 when the viral load is ≥ 104, 105 and 106 copies/mL respectively. This result is identical to that found in our study, which showed that a high viral load ≥ 2000 IU/mL is associated with a risk of fibrosis progression with a p value of 0.001 (Table 2).
Alanine aminotransferase (ALT) levels were measured in all patients, as follows: ALT normal in 50% of cases (n=100), ALT greater than twice normal in 30% of cases (n=60) and ALT greater than twice normal in 20% (n=40). This high frequency of cytolysis in our study could be explained by the fact that in Black Africa the causes of hepatic cytolysis are numerous and interrelated, and suggest viral, drug and toxic causes [27].
In our series, abdominal ultrasound revealed:
− Normal liver in 75% (n=150)
− Hepatic steatosis in 20% (n=40)
− Non-decompensated cirrhosis liver in 5% (n=10)
This result is similar to that reported by Bamouni et al. [28] who noted an ultrasound hepatic steatosis of 30.38%.
Our patients' histories included: drug therapy (22.5%; n=45), jaundice (18%, n=36), diabetes (10%, n=20), hypertension (7.5%, n=15), dyslipidaemia (6%, n=12), blood transfusion (4.5%, n=9), dental treatment (4%, n=8) (Table 1). Some of these antecedents were associated with a statically significant risk of fibrosis progression (Table 4).
Conclusion
Viral hepatitis B remains a worldwide public health problem. The purpose of assessing fibrosis in patients with HBV is to diagnose moderate or severe fibrosis in order to establish a therapeutic indication, to initiate screening for complications such as HCC or portal hypertension, and finally to assess the impact of any associated co-morbidities: NASH, alcohol, autoimmunity, metabolic syndrome, Delta, HCV or HIV co-infections, which are risk factors for progression of liver fibrosis. Fibroscan/CAP is a painless test that is very well accepted by the patient and can therefore be easily repeated, making it possible to monitor the development of fibrosis over time. Despite recent advances in antiviral therapies, the treatment of chronic hepatitis B is difficult and costly. Preventing HBV infection by systematic vaccination remains the best option for reducing morbidity and mortality from liver failure and liver cancer.
Declaration for human rights
The hospital consented to the use of data from patients who were seen in the department. The study was approved by the hospital's ethics committee and the principles of the Declaration of Helsinki were followed.
References
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus J Hepatol. 2017; 67: 370-398.
- GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study Lancet Gastroenterol Hepatol. 2022; 7: 796-829.
- Ott JJ, stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBs Ag seroprevalence and endemicity, Vaccine. 2012; 30: 2212-
- Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral WHO. 2016.
- Loua A, Diallo MB, Magassouba Séroprévalence de l'hépatite B chez les donneurs de sang en Guinée; Med Trop: revue du corps de santé colonial. 2005; 65: 396-397.
- Baldé NM, Camara A, Kourouma K, et al. Characteristics cliniques de la Séroprévalence à l'hépatite B et au VIH chez 248 Diabétiques à Conakry en Guinée. Afrique Noire A. 2007; 54: 174-178.
- Bravo A, Shet SG, Chopra S, et al. Liver biopsy. N Engl J 2001; 334: 495-500.
- Leroy V, Hilleret Evaluation of hepatic fibrosis. Hepato- Gastro. 2005; 12: 251-259.
- Chalasani N, Younossi ZM, Lavine JE, et al. The diagnosis and management of non alcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Hepatology. 2018; 67: 328-357.
- Brener Transient Elastography for Assessment of liver Fibrosis and Steatosis: An Evidence- Based Analysis. On Health Technol Assess Ser. 2015; 15: 1-45.
- Sandrin L, Fourquet B, Hasquenoph JM, et Transient elastography: a new non-invasive method for assessment of hepatic fibrosis. Ultrasound in Medicine and Biology. 2003; 29: 1705-1713.
- Milligo GRC, Samandoulougou A, Yaméogo NV, et Metabolic Syndrome in hypertensive patients in the cardiology departement of Yalgado Ouedraogo University Hospital of Ouagadougou, Burkina Fasso. Pan Afr Med J. 2014; 19: 290.
- Assellah T, Lada O, Boyer N, et al. Treatment of hepatitis B. Gastroenterol Clin Biol. 2008; 32: 749-768.
- Mongo-Onkouo A, Ahoui Apendi CP, Mimiesse Monamou JF, et al. Financial cost of treating chronic viral hepatitis B and C at Brazzaville University Hospital (Congo). Health Dis. 2019; 20: 46-49.
- Diallo MS, Youssouf O, Yaogo A, Diallo D, et Evaluation of Hepatic Fibrosis and Hepatic Steatosis by Pulse Elastography (FIBROSCAN) in Asymptomatic Patients about 170 Cases at the Donka CHU National Hospital in Conakry. Open Journal of Gastroenterology. 2024; 14; 125-138.
- Seto WK, hui RWH, Mak LY, et al. Association Between Hepatic steatosis, Measured by Controlled attenuation Parameter, and Fibrosis Burden in chronic Hepatitis B. Clin Gastroenterol 2018; 16: 575-583.
- Choi HSJ, Brouwer WP, Zanzjir WMR, et al. Nonalcoholic steatohepatitis Is Associated with Liver-Related Outcomes and all-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020; 71: 539-548.
- Chen Y, Fan C, Chen Y, et al. Effect of hepatic steatosis on the progression of chronic hepatitis B: a prospective cohort and in vitro Oncotarget. 2017; 8: 58601-58610.
- Wang CY, Lu W, Hu DS, et Diagnostic value of controlled attenuation parameter for liver steatosis in patients with chronic hepatitis B. World J Gastroenterol. 2014; 20: 10585- 10590.
- Fattovich G. Natural history and prognosis of hepatitis B. semin Liver Dis. 2003; 47-58.
- Ganem D, Prince AM. Hepatitis B virus-natural history and clinical N Engl J Med. 2004; 350: 1118-1129.
- Mallem L, Safir A, Amani N, et al. Risk factors for hepatic fibrosis in chronic B virus carriers followed at Oran University Algerian Journal of Health Sciences. 2019; 1: 21-26.
- Pascale G, Ariane M, Sophie L. Role of myofibroblasts in liver Hépato-Gastro. 2005; 12: 511-523.
- Sawadogoa BB, diba B, Calès Pathophysiology of cirrhosis and its complications. Réanimation 2007; 16: 557-556.
- Wong GL, Chan HL, Yu Z, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression inpatients with chronic hepatitis B- a prospective cohort study with paired transient elastography Aliment Pharmacol Ther. 2013; 39: 883-893.
- Huo TI, Wu JC, Hwang SJ, et al. Factors predictive of liver cirrhosis in patients with chronic hepatitis B: a multivariate analysis in a longitudinal study. Eur J Gastroenterol Hepatol. 2000; 12: 687-693.
- Diallo MS, Wann TA, Diallo D, et al. Autoimmune hepatitis complicated by cirrhosis: about an observation at the Conakry University Jaccr Africa. 2023: 7: 65-70.
- Bamouni YA, Cissé R, Diallo O, et al. Etiological Factors associated with hepatic steatosis discovered accidentally by ultrasound in Ouagadougou, Burkina Fasso. J Afr Imag Médicale. 2012; 4: 21-30.