Sagacity to Decode Cardiogenesis Mystries: Etiological Perspective of Cardiovascular Malformations
Author(s): Abdullah Alabdulgader MD, DCH (Dublin), DCH (Edinb), MRCP (UK), ABP, FRCP (Edinb), Ali S Alhazmi M.Sc (Pharmacology) and Lamees A Alabdulgader MD
1Chief Cardiologist & Founder, Prince Sultan Cardiac Center, Alhasa, Saudi Arabia.
2Regional DPIC, Complementary and Alternative Medicine, Northern Zone, Saudi Arabia.
3Fellow of Family and Community Medicine, College of Medicine, Imam Abdulrhman Bin Faisal University, Dammam, Saudi Arabia.
*Correspondence:
Abdullah Alabdulgader, Chief Cardiologist & Founder, Prince Sultan Cardiac Center, Alhasa, Saudi Arabia.
Received: 07 Dec 2021; Accepted: 28 Dec 2021; Published: 02 Jan 2022
Citation: Alabdulgader A, Alhazmi AS, Alabdulgader LA. Sagacity to Decode Cardiogenesis Mystries: Etiological Perspective of Cardiovascular Malformations. Cardiol Vasc Res. 2022; 6(1): 1-9.
Keywords
The Birth of etiological studies in the current era: Quarter-Century of our life has been passed while we are still diving in the science of human cardiogenesis and Cardiovascular Malformations. The far away, great dream in the horizon is establishment of cardiogenesis knowledge capable of leading us to intervene and abort the developmental derangement leading to congenital cardiovascular malformation in human species. Knowledge of the epidemiology of congenital heart disease is the basis on which investigative efforts will emerge to identify the causes of cardiac dysmorphogenesis and afford opportunities to prevent them. Anatomical and pathophysiological classification of congenital heart disease was found to be useful in clinical practice but useless or even misleading for investigating etiological factors of congenital heart diseases. We adopted an etiological perspective of congenital heart diseases which in many directions pose no relation to the current anatomical and pathophysiological classification. For example, bicuspid aortic valve which prove to be very common in all races with incidence of 3-5% of world populations, carries no clinical significance until hemodynamically significant gradient developed with aging to yield left ventricular outflow tract obstruction at valvular origin. On the other hand, in our new etiological perspective bicuspid aortic valve is strong developmental land mark for spectrum of congenital malformations of the heart ranging from simple aortic valve disease to the extreme Shones complex with sever hypoplasia of the left ventricle, mitral valve, aortic valve and the aortic arch and branches hypoplasia. Presence of simple spectrum of Shones disease in one family member may point to sever diseases in other family members with mutations in the gene encoding myosin and other shared mutations. On the other hand, clinical diseases with exactly same pharmaceutical treatment plans like muscular ventricular septal defect and supracristal ventricular septal defect, carries two different developmental pathways in our new etiological perspective. We in the current era need to base our approach to investigate cardiogenesis secrets, on embryologic terms but not the pathophysiological grouping which was established for clinical and therapeutic puposes. Viewing cardiac defects from mechanistic rather than strictly anatomical perspective is the true call for wisdom if we are targeting prevention of cardiac dysmorphogenesis in human.
Ferencz C, et al in the early 1980s heralded the onset of the necessity of etiological perspective of Congenital Heart Diseases (CHD). The Baltimore-Washington Infant Study is a regional epidemiologic study of congenital heart disease among Infants born in the study area between1981 and 1989. Around 4000 infants had a diagnosis of congenital heart disease confirmed in the first year of life by echocardiography, cardiac catheterization, cardiac surgery, or autopsy [1]. We in the Kingdome of Saudi Arabia were leading in this direction since 1997 where we established the first incidence of congenital heart disease in the region built on meticulous and rigorous examination of congenital heart disease affected infants between March 1997 to February 2000.Our reported incidence of CHD was among the highest in the era ever, with occurrence of 10.67 CHD affected infant per 1000 live birth [2,3]. Prevalence studies used to count old and new cases while incidence is counting only new cases at that year of the study. The Baltimore Washington Infant study group reported prevalence rate was 3.7/1,000 livebirths for all cases and 2.4/1,000 livebirths for cases confirmed by invasive methods only. Diagnosis- specific prevalence rates of congenital heart disease are compared with those of eight previous case series. Changing diagnostic categorizations in the time span covered and methodological differences resulted in great variation of the data. Using the same discovery methods The New England Infant Cardiac Program showed similar occurrences of major morphologic abnormalities, suggesting that these are stable basic estimates in the eastern United States. For all case series, the rate of confirmed congenital heart disease was approximately 4/1,000 livebirths over the 40- year time span. Our reported high incidence in Saudi Arabia was brought to the attention of decision makers in the country where we established the national and regional project named: (Genetic and Environmental Risk Factors of Congenital Heart Diseases in Kingdom of Saudi Arabia), where we adopted a true etiological perspective. A case-control study of congenital heart defects (CHD) in Saudi live born infants 2005-2010 was finally completed. Hospitals, primary health care centers, pediatric cardiology centers in KSA concerned with CHD are participants in the project constituting a unique national sample. The main objective of the project was to shed light on probable environmental and genetic risk factors implicated in the etiology of Congenital Heart Disease (CHD) in Saudi population. Cases were defined as infants born alive in 2005-1010 to parents who were residents of the study area. Disease status was defined as CHD present at birth (coded 1) else, it is absent for the (coded 0). CHD diagnosis confirmed before 1 year of age by pediatric cardiologists, according to hierarchical classification system. Questionnaires were expanded to include a detailed inquiry about exposures to various environmental and genetic factors. 7327 questionnaires (4465 cases + 2862 control) from all over KSA were collected. More control records were intentionally accumulated to balance case-control ratio to at least 1:1. Parents are interviewed about a wide range of genetic, physiological, medical and environmental factors that occurred during and before the pregnancy, assessing exposures at home, job and other sites. Data sheets were designed to filter and encode the information from the questionnaires and qualitatively controlled (Figure 1). Original data was strictly maintained and adhered to standardization protocol. The database records and variables amount to 7327*412 information matrix maintained (equivalent to around 3000,000 statistical variables) within a relational data- base system established on oracle-SQL server. Connectivity to SAS package was maintained through ODBC driver. For the data management and statistical analysis SAS package is implemented, applying conditional logistic regression and auto calls macros according to the data set and analysis purpose. A stratified conditional logistic regression analysis (with the same modeling flexibility as an unconditional analysis), taking into account the correlation structure attributable to matching is the most efficient way to control for a known confounder. Matching is facilitated by specialized macros particularly developed for fine matching and stratification. Therefore, a modeling technique that correctly incorporates the matched nature of the data is highly recommended and adopted for this matched case-control study. All the variables were categorized to cover various infant, maternal and paternal aspects. Index variables like socio-economic score and genetic score were derived from a combination of several basic factors. Maximum likelihood method is the algorithm of choice for most of the analysis. Descriptive statistics were reported as mean value and 95% Confidence Interval (CI). Association between any two variables was tested using Chi-square test. The general strategy of the analysis was two stage logistic regression performed as univariate analysis followed by multivariate analysis to provide the estimated effect of each risk factor. Demographic data stratification precludes heterogeneity across a wide case-control sub sample. Results suggest an association of specific maternal, paternal, nutritional and cosmetic factors during the first trimester with certain CHD. These results raise new questions about the possible epidemiologic association of certain heart defects with potential risk factors that warrant new, carefully targeted investigations.
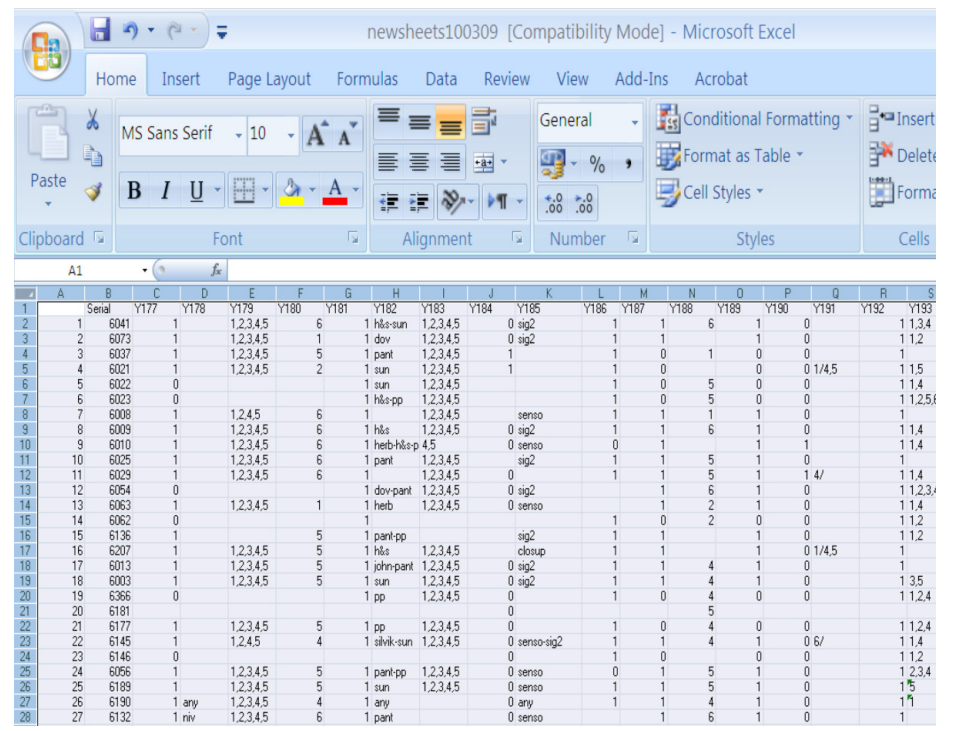
Figure 1: Data-entry sheet for various patients.
Chronobiology of embryological Cardiac events
The most difficult historical question related to Cardiac morphogenesis in human is the timing of the intrauterine insult considering the extensively and complex cascade of delicate events,error in any, will yield congenital heart disease. In order to perceive the abnormal cardiac development, knowledge of normal development is cruicial. Detailed information of the complex creature of human heart was obtained using microscopic sectioning and other more recent tecniques. Evaluation of human embryo specimen to determine exact time of ovulation or fertilization is difficult. Embryologists used to consider the appearance of prominent structures to establish approximate age in the early embryo. Prominent paired blocks of tissue appeared dorsally near the midline in the end of the third week is called somite [4]. Somites are increasing at fairly constant rate and used in the early embryogenesis to estimate age as seen in table 1.

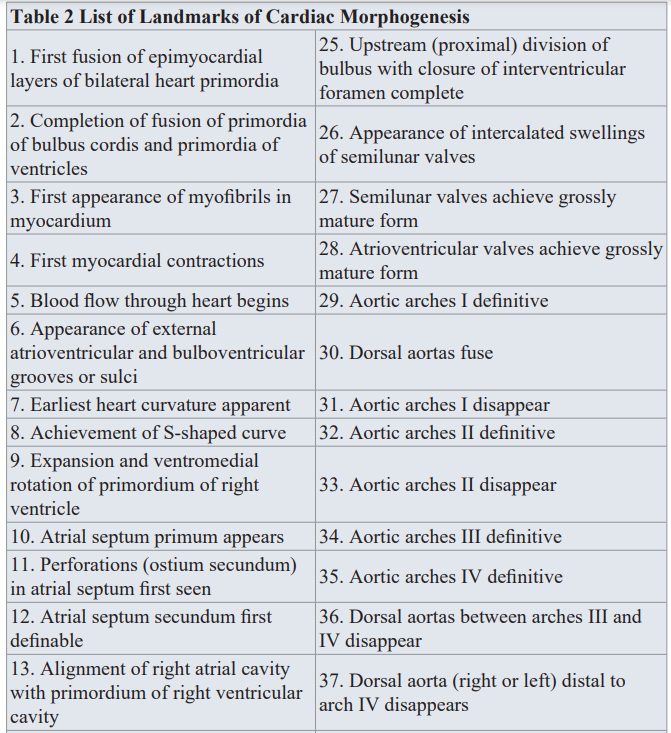
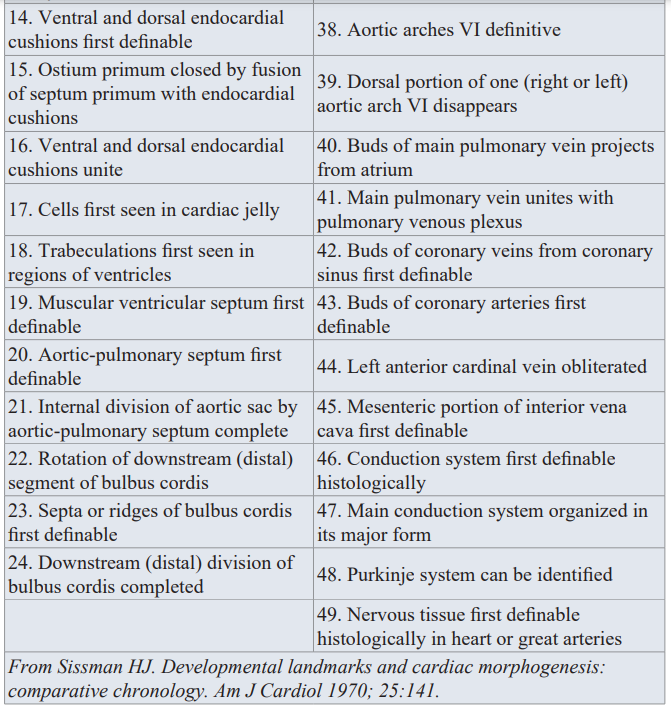
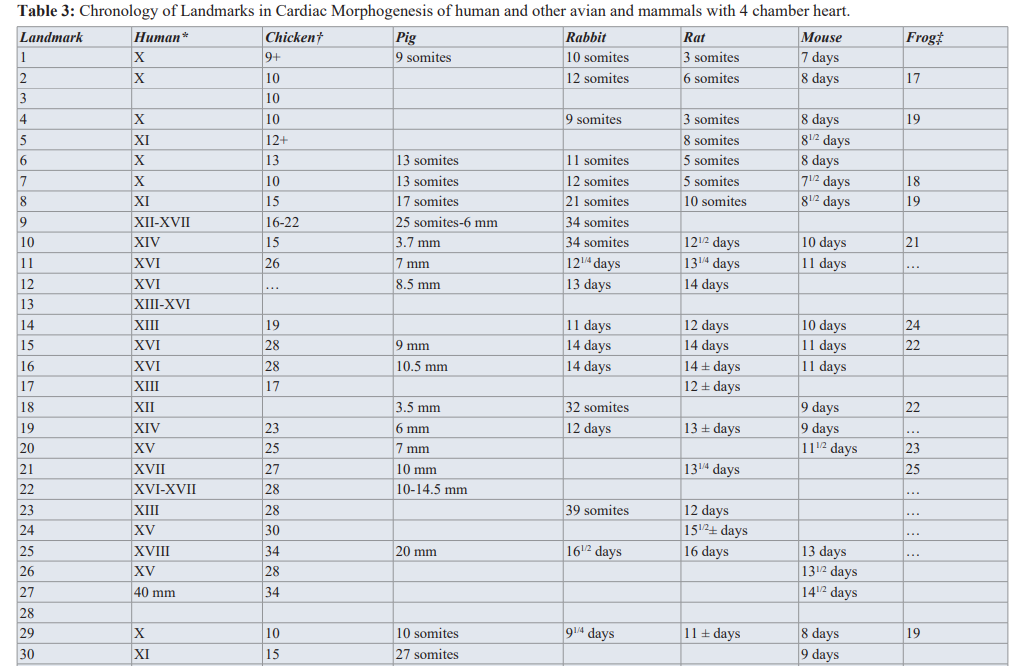
Determination of the exact time of the insult of specific morphogenetic step(s) during heart embryogenesis has been question of major debate between us in the cardiogenesis arena. Knowledge of specific lesion timing is of increasing importance in cases of multiple intracardiac lesions, syndromes and in the presence of extracardiac anomaly (ECA).Steps leading to the formation of the four chamber heart are too complex with overlapping and intricate nature. We in our etiological perspective project, adopted cardiac morphogenesis land marks and Chronology Landmarks of Cardiac Morphogenesis From Sissman HJ (Developmental landmarks and cardiac morphogenesis: comparative chronology. Am J Cardiol 1970; 25:141), as seen in Table 2 and Table 3.
Cinical studies, imagining techniques, autopsy and molecular cardiology to validate etiological perspective derived data Decoding the secrets of cardiogenesis can be facilitated by different methods with diversity of approaches. Clinical evidence of common molecular and signaling pathways between heart and other organs is evident in the context of Extra Cardiac Anomalies (ECA). We in the clinical arena can support higher yield of discoveries in the molecular genetics arena with special approach aiming the delineation of specific cardiac lesion association with specific extra cardiac lesions. Addition of associated etiological risk factor, either genetic or environmental will bring us closer to the cause effect relation ship of the specified lesion. We investigated
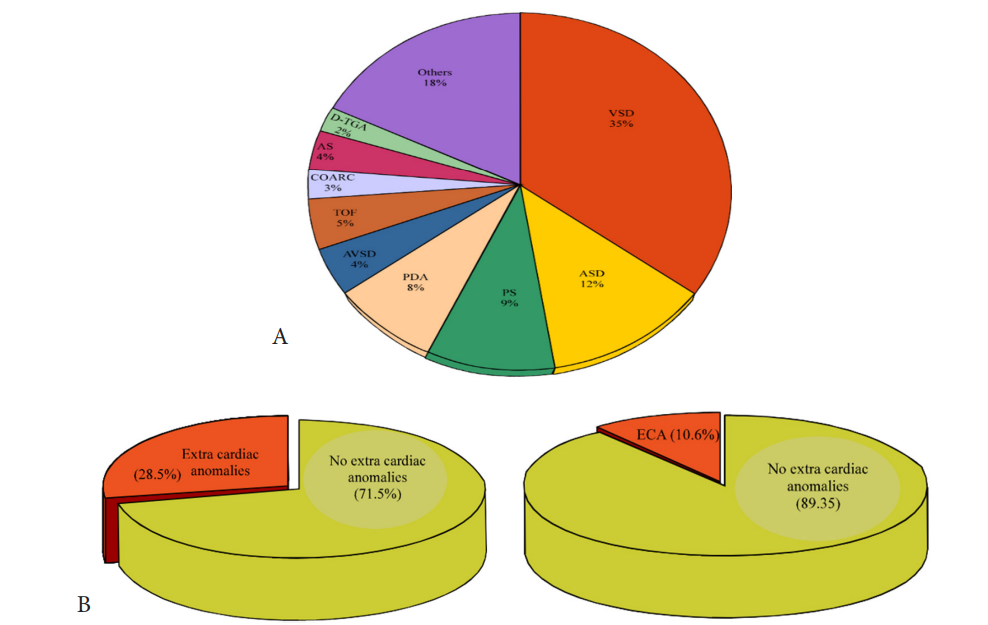
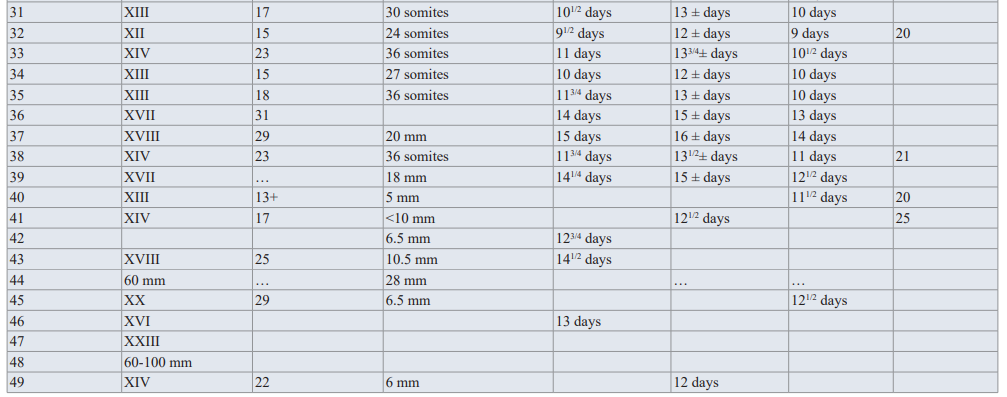
Figure 1: A is a pie diagram demonstrating the percentage of each congenital heart disease of the 10 most common lesions. The two comparable pie diagrams in B illustrate the significance of associated ECA with congenital heart diseases, suggesting associated molecular pathways between cardiogenesis and other systems morphogenesis.(Courtesy of Abdullah Alabdulgader, Extra cardiac anomalies (ECA) in 2020 subjects with congenital cardiovascular malformation (CCVM) and control: Etiological perspective. Journal of Clinical and Experimental Cardiology).
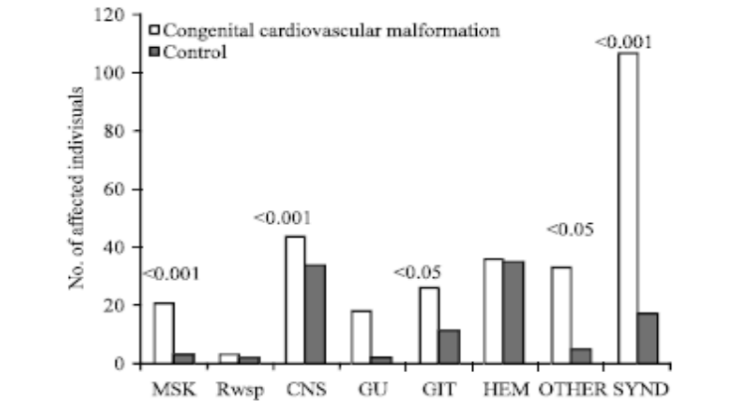
Figure 2: Bar diagram comparing cases and controls for the different systems with corresponding p-value, MK: Musculo-skeletal, Resp: Respiratory system, CNS: Central nervous system, GU: Genito-urinary, GIT: Gastro-intestinal tract, HEM: Hematological, SYND: Syndrome. (Courtesy of Abdullah Alabdulgader, 2012. Extra Cardiac Anomalies (ECA) in 2020 Subjects with Congenital Cardiovascular Malformation (CCVM) and Control: Etiological Perspective. Journal of Medical Sciences, 12: 29-36).
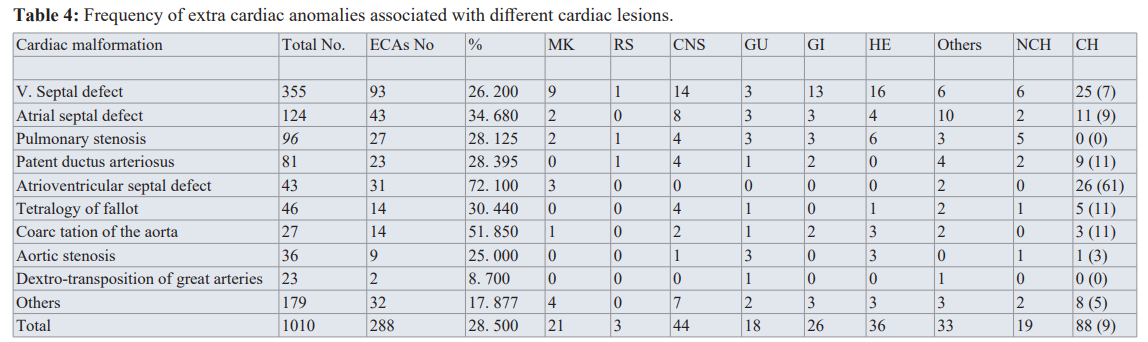
MK: Musculo-skeletal, RS: Respiratory system, CNS: Central nervous system, GU: Genito-urinary, GI: Gastro-intestinal, HE: Hematologic al, NCH: Non-chromosomal, CH: Chromosomal figures in braces show the percentage for each) .(Courtesy of Abdullah Alabdulgader, Extra cardiac anomalies (ECA) in 2020 subjects with congenital cardiovascular malformation (CCVM) and control: Etiological perspective. Journal of Clinical and Experimental Cardiology).
1010 cases of CHD and 1010 of control. The significance of higher cases of CHD associated with ECA is evident (28.5% in cases versus 10.6 in control) suggesting associated molecular pathways between cardiogenesis and other systems morphogenesis (Figure 1). At more delinated perspective looking at specific CHD type association with specific extra cardiac systems is shown in table 1 With reverse perspective we investigated comparison of cases and controls for the different systems with corresponding p-value as seen in Figure 2. This multiple angel’s perspective is thought to be an excellent modality to solve complex puzzles like dysmorphogenesis in human species [5,6].
In the recent years molecular genetics has significant contribution to our understanding of heart development But due to the nonspecific role of genetic mutations in cause effect relationship to generate congenital heart disease, epidemiological studies with risk factors orientation are thought by us and others to be the gold standard to come closer to the absolute fact in cardiac dysmorphogenesis. Researchers in the field of molecular genetics (Srivastava D and colleagues from Gladstone Institute of Cardiovascular Disease, San Francisco, CA 94158, USA) created true revolution in our molecular cardiology understanding and its contribution to genesis of congenital heart lesions. They demonstrated that somatic cells, such as fibroblasts, can be dedifferentiated into pluripotent stem cells by nuclear transfer [7] or with defined transcription factors [8]. Direct reprogramming of fibroblasts into the chief functional cells of different organs, including cardiomyocytes (CMs), neurons, hepatocytes, hematopoietic cells, and endothelial cells, has been accomplished by their group. With this level of understanding the dream of regenerative medicine in human history is revolutionized [9]. In particular, cardiac fibroblasts represent a large pool of cells in the adult heart [10] and thus may provide a reservoir of cells from which to generate new CMs through epigenetic reprogramming. In 2010, Srivastava and colleagues reported that mouse cardiac and dermal fibroblasts could be directly reprogrammed into induced CM-like cells (iCMs) in vitro by a combination of three developmental cardiac transcription factors, Gata4, Mef2c, and Tbx5 (GMT) [11]. Since then, other labs around the world have reported success in reprogramming mouse fibroblasts into iCMs with similar cocktails of reprogramming factors [12-16]. Srivastava D and others have also directly converted human cardiac and dermal fibroblasts into cardiac cells [17-19]. Several review papers published from different labs illustrate the potential and challenges of this new avenue for cardiac regenerative medicine [20-26] which may contribute to revolutionary treatment for CHD in utero or early infancy.
With all this revolutionary approach to decode the secrets of heart development, the role of epidemiological risk factors based projects remains highly important.The molecular mechanisms and reprogramming approaches to cardiovascular disease and its contribution to developmental biology, cardiogenesis and regenerative medicine remains of paramount importance and is an excellent complementation if guided by large scale epidemiology of congenital heart diseases.
In addition, anatomical findings either by echocardiography, MRI or autopsy remains an important priority in the field. In the case of stillborn infants, the incidence of congenital heart defects was found in large number of studies to be 10 times higher than that of live births. We reported an autopsy case for two weeks old native Canadian with unique combination of laryngotracheoesophageal cleft (LTEC) type 3 and Double Outlet Right Ventricle (DORV) as the first combination described in the medical literature [27]. Our discovery of the cardiac and other systems combinations in this autopsy guided us to diagnose combination lesions and suggest the stage of its development in the intrauterine life which is critical clue to decode cardiogenesis in human species. The association of DORV with subpulmonic VSD suggests the rule of widespread insult during the early embryogenesis. The rule of genetic contribution to the development of LTEC and CHD is strongly supported by the higher frequency of both lesions with syndromes and aneuploidy. Tyler [28] documented an incidence of CHD in 11% of LTEC individuals. We observed that a significant portion
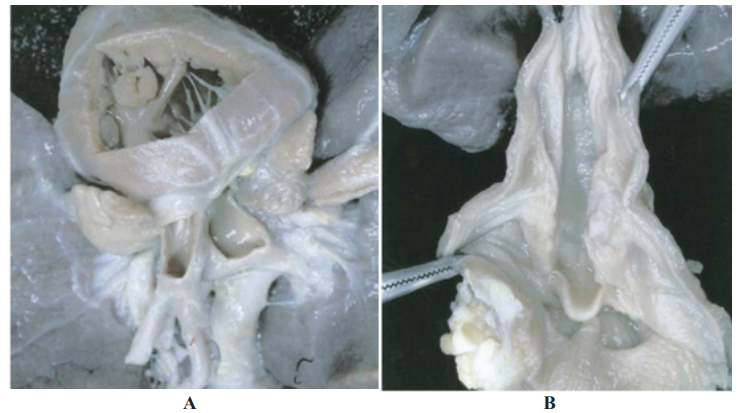
Figure 2: Autopsy remains golden contribution to the field of etiological cardiogenesis. Unique combination of Double Outlet Right Ventricle(A) and Laryngotracheoesophageal cleft type 3 (B). (Courtesy:Abdullah Alabdulgader. Annals of Diagnostic Pathology 9 (2005) 323 – 326).
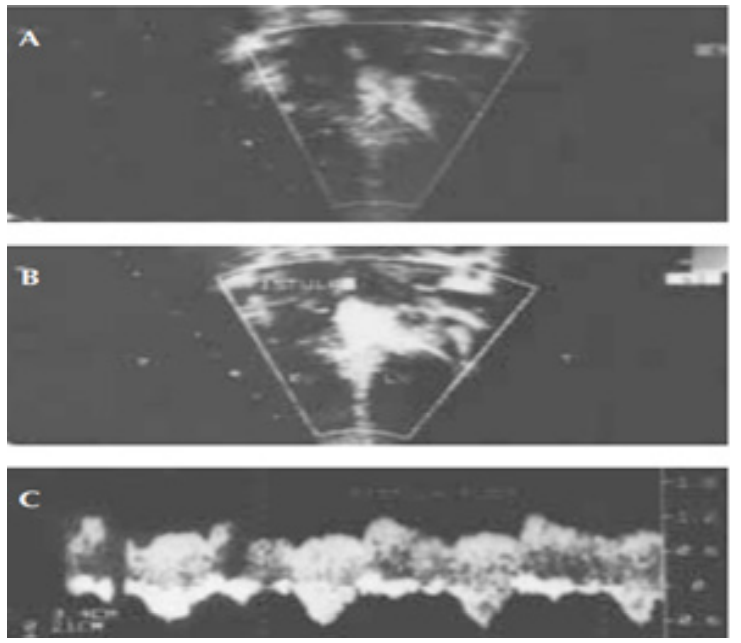
Figure 3: Careful echocardiography with meticulous doppler at outlet portion of septal shunts led to diagnosis of congenital coronary artery fistula after tetralogy of fallout repair. A is 2D frame of the coronary artery fistula. B illustrate the fistula shunt and C is documenting continuous pulse wave doppler flow of coronary artery with diastolic accentuation (Courtesy: Abdullah Alabdulgader, Asian Cardiovasc Thorac Ann 2002;10:339–41).
Of CHD occurring in association with LTEC is of the conotruncal group, including tetralogy of Fallot and arch anomalies. In this case report, we described another type of conotruncal anomalies in association with LTEC, which is DORV. More specific linkage between LTEC and conotruncal anomalies can be established by shedding more light on the cephalic neurocristopathy (CNCP), which is an abnormality of neural crest differentiation, and its contribution to both tracheoesophageal septum related lesions and conotruncal anomalies. Patients with CNCP present striking pattern of associated cardiovascular anomalies. Francesco et al were able to create linkage between esophageal atresia and CNCP-related conotruncal anomalies [29]. Tracheoesophageal fistula and esophageal atresia are known to share common pathogenesis with LTEC because both lesions are the consequence of tracheoesophageal septum defect [28].
On the other hand, the presence of difficult to diagnose lesions which may contribute minimal or non to clinical picture but carries significant contribution to etiological researchers, might be facilitated by echocardiography with colour flow mapping and doppler studies. We reported Coronary artery fistula (CAF) manifested only after successful Tetralogy Of Fallout (TOF) surgical repair [30]. The discovery of this hidden lesion by echocardiographic color flow mapping and doppler studies change the etiologic timing of the intrauterine insult leading to this CHD to earlier periods than expected for Tetralogy Of Fallout (TOF) (Figure 3).
Conclusion
Cardiac development in human is highly sophisticated developmental process characterized by large number of mechanisms guided by genetic, epigenetic and intricate signaling network. The road map to disclose this mysterious and miraculous developmental operations must be guided by etiological studies based on extensive epidemiological search for genetic and environmental risk factors. This epidemiological derived knowledge should be supplemented by clinical, imagining and molecular techniques as well as autopsy studies to shed more light on the associated defective pathways and the stage of arrest of development. Our congenital heart disease project in Arabian Peninsula is a successive step towards disclosing the mystery of cardiac dysmorphogenesis with the ultimate aim to prevent congenital heart diseases in human species.
References
- Ferencz C, Rubin JD, McCarter RJ, et al. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol. 1985; 121: 31-36.
- Alabdulgader A. Congenital heart disease in 740 subjects: epidemiological aspects. Annals of tropical paediatrics. 2001; 21: 111-118.
- Alabdulgader A. Congenital heart disease in Saudi Arabia: current epidemiology and future projections. East Mediterr Health J. 2006; 12: 157-167.
- The science and practice of pediatric cardiology, second edition: Volumes I and II: Edited by Arthur Garson Jr, J Timothy Bricker, David J. Fisher, and Steven R. Neish Williams & Wilkins, Baltimore (1998) 3,200 pages, ISBN: 0-683-034 17-0. Clin Cardiol. 1999; 22: 54.
- Abdullah A. Alabdulgader. Extra Cardiac Anomalies (ECA) in 2020 Subjects with Congenital Cardiovascular Malformation (CCVM) and Control: Etiological Perspective. Journal of Medical Sciences. 2012; 12: 29-36.
- Alabdulgader A. Extra cardiac anomalies (ECA) in 2020 subjects with congenital cardiovascular malformation (CCVM) and control: Etiological perspective. Journal of Clinical and Experimental Cardiology. 2016.
- Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962; 10: 622-640.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126: 663-676.
- Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol. 2012; 14: 892-899.
- Snider P, Standley KN, Wang J, et al. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009; 105: 934- 947.
- Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010; 142: 375-386.
- Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA- mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012; 110: 1465- 1473.
- Protze S, Khattak S, Poulet C, et al. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012; 53: 323-332.
- Song K, Nam YJ, Luo X, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012; 485: 599-604.
- Addis RC, Ifkovits JL, Pinto F, et al. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J Mol Cell Cardiol. 2013; 60: 97-106.
- Christoforou N, Chellappan M, Adler AF, et al. Transcription factors MYOCD, SRF, Mesp1 and SMARCD3 enhance the cardio-inducing effect of GATA4, TBX5, and MEF2C during direct cellular reprogramming. PLoS One. 2013; 8: e63577.
- Fu JD, Stone NR, Liu L, et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 2013; 1: 235-247.
- Nam YJ, Song K, Luo X, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013; 110: 5588-5593.
- Wada R, Muraoka N, Inagawa K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA. 2013; 110: 12667-12672.
- Yi BA, Mummery CL, Chien KR. Direct cardiomyocyte reprogramming: a new direction for cardiovascular regenerative medicine. Cold Spring Harb Perspect Med. 2013; 3: a014050.
- Srivastava D, Berry EC. Cardiac reprogramming: from mouse toward man. Curr Opin Genet Dev. 2013; 23: 574-578.
- Qian L, Srivastava D. Direct cardiac reprogramming: from developmental biology to cardiac regeneration. Circ Res. 2013; 113: 915-921.
- Nam YJ, Song K, Olson EN. Heart repair by cardiac reprogramming. Nat Med. 2013; 19: 413-415.
- Addis RC, Epstein JA. Induced regeneration-the progress and promise of direct reprogramming for heart repair. Nat Med. 2013; 19: 829-836.
- Muraoka N, Ieda M. Direct reprogramming of fibroblasts into myocytes to reverse fibrosis. Annu Rev Physiol. 2014; 76: 21- 37.
- Nakanishi T, Markwald RR, Baldwin HS, et al. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology [Internet]. Springer; 2016.
- Alabdulgader A, Patten D, Harder J, et al. Laryngotracheoesophageal cleft type 3 and double outlet right ventricle: unique combination. Annals of diagnostic pathology. 2005; 9: 323-326.
- Tyler DC. Laryngeal cleft: report of eight patients and a review of the literature. Am J Med Genet. 1985; 21: 61-75.
- Morini F, Cozzi DA, Ilari M, et al. Pattern of cardiovascular anomalies associated with esophageal atresia: support for caudal pharyngeal arch neurocristopathy. Pediatr Res. 2001; 50: 565-568.
- Alabdulgader A. Noninvasive Diagnosis of Coronary Artery Fistula after Cardiac Surgery. Asian Cardiovascular and Thoracic Annals. 2002; 10: 339-341.