Six Years of Neuro-Navigation Assisted Brain Tumor Surgery North Central Nigeria: Progress and Challenges
Author'(s): Ugwuanyi UC1*, Anigbo AA1, Nwaribe EE1, Morayo MM2, Jamgbadi SS, Jubril PG3, Ayogu OM1, Okpata CI1, Ekpendu I1, Okafor N1, Onobun DE1, Mordi C1, Arua C4 and Nwachukwu E4
1Department of Surgery, National Hospital Abuja and Wellington Hospitals Abuja, Nigeria.
2Department of Anesthesia, National Hospital Abuja and Wellington Hospitals Abuja, Nigeria.
3Department of Neuropathology, National Hospital Abuja,Nigeria
4Department of Radiation Oncology, National Hospital Abuja,Nigeria
*Correspondence:
Ugwuanyi Ugochukwu Charles MD, FMCS, FICS, FACS, Chief Consultant and Head of Neurosurgery, National Hospital Abuja, Nigeria, Wellington Neurosurgery Center Abuja, Tel: +234 7036842976.
Received: 27 Feb 2023; Accepted: 01 Apr 2023; Published: 05 Apr 2023
Citation: Ugwuanyi UC, Anigbo AA, Nwaribe EE, et al. Six Years of Neuro-Navigation Assisted Brain Tumor Surgery North Central Nigeria: Progress and Challenges. Cancer Sci Res. 2023; 6(1): 1-7
Abstract
Introduction: Cranial operations in general have been historically driven by sound anatomical navigation with tremendous success. However, due to the unending desire for perfection, computerized neuro-navigation-assisted cranial surgery has found global usefulness, especially in neuro-oncological services. Our initial experience and challenges with this technology was already published three years ago but it becomes expedient for an update.
Aims and Objectives: To report a six-year experience with computerized neuro-navigation assistance in our neuro-oncology surgeries and services. Methodology: Retrospective review of all consecutive cases involving computer-assisted neuro-navigation diagnostic and resective brain tumor operation over a six-year period (January 2016-December 2021). Main study parameters: Clinical diagnostic procedures, operations, histological diagnosis, adjuvant treatments. Data were analyzed using simple descriptive statistics, and results were presented accordingly.
Results: Total number of cases 111, Males 70 and Females 41 (M: F = 1.7:1). Age ranges from 8 months to 80 years. The commonest presentation was headache, nausea, and vomiting. Pre-op diagnosis: Intra axial tumors 70/111 (63%), Extra axial tumors 41/111 (36%). Operations: Resection/Debulking of tumor 86/111 (77,4%), with complete EOR of 65/86 (75%). Diagnostic Biopsy 68/111 (61%) with target precision of 100%. Common Histology were Gliomas 50/111 (45%), Meningiomas 41/111 (36.9%), and Metastatic 15/111 (13.5%). Adjuvant treatments and follow-ups were advised accordingly.
Conclusions: With computerized neuro-navigation assistance, a wider spectrum of brain tumors were more confidently operated, and adjuvant treatments were easily deployed in line with a precise histological diagnosis for improved neuro-oncology services. But this success was at a huge cost of adopting measures to overcome some inevitable challenges.
Keywords
Introduction
By definition, neuro-navigation is the set of computer-assisted technologies used by neurosurgeons to guide or "navigate” within the confines of the skull or vertebral column during surgery [1]. Cranial operations in general have been historically driven by sound anatomical navigation with tremendous success. However, due to the unending desire for perfection, computerized neuro- navigation assisted cranial surgery has found global usefulness, especially in neuro-oncological services.
Despite development of many lesion localizing imaging techniques including angiography, MRI, CT scan, sonography, and frame base stereotaxy, a more accurate and near real time localizing technique is still desired in modern neurosurgery [2]. Stealth guided neuronavigation is one of the techniques that fit into this desire but perhaps intra-operative imaging updating with ultrasound, MRI inces closer to higher accuracy.
The basic principle in neuro-navigation is to establish early an accurate transformation matrix which creates a linkage between digital image data and anatomical structure, and therefore, to provide increasing 3-D orientation [3]. As previously reported [4], co-registration of previously acquired imaging coordinates and actual physical anatomical coordinates provides a means of synchronizing both and forms the backbone for neuro-navigation as well as other stereotactic procedures.
The basic procedure for neuro-navigation involves the following steps: 1-Establishment of physical coordinates which can be conventionally frame-based using stereotactic frames or frameless using fiducial markers or surface landmarks. 2-Establishment of imaging coordinates with any or combination of the following imaging modalities – MRI, CT, PET, single-photon emission CT, X-ray, functional MRI etc. 3- Registration of the imaging coordinates on the computerized systems of the Navigation machine 4-Co-registration of the imaging coordinates and the actual physical anatomical space which forms the backbone of neuro-navigation accuracy. 5-Surgical planning to define surgical entry points, surgical corridor and trajectory to surgical targets. 6-Navigation, which continues throughout the surgical process- diagnostic biopsy or tumor excision/debulking.
A navigation error margin of 2-4 mm may be tolerable in white matter dissection but definitely not in targeting specific nuclei in functional neurosurgery and in modern stealth machines an inbuilt error control margins will not allow any navigation when the error margin becomes more than 5 mm [5]. Intra-operative brain deformation (brain shift) is a natural and inevitable source of error in image-guided neuro-surgery systems [6] but there have been recent attempts to deploy real time ultrasound as a less cumbersome alternative to intraoperative MR imaging which is also a more expensive system [7].
It is important to note the main clinical utilities of Neuro-navigation in modern neurosurgery especially localization of small intracranial lesions, skull-base surgery, intra cerebral biopsies, intracranial endoscopy, functional neurosurgery and spinal navigation [8] but in this current report the focus is on brain tumors.
From onset, the brain tumor management protocol of the study center incorporated neuro-navigation for quality assurance purposes. In addition, in view of the complexity of the mandatory requirements, the neuro-oncology services evolved slowly because all emerging challenges had to be overcome in order to achieve perfection. The details of these challenges are discussed in this paper as an integral part of the successes we recorded already in the application of this technology in our neuro-oncology surgical services. Unfortunately, this study does not compare with any previous data, because non existed before the neuro-oncology protocols were set up following the installation of navigation system. This article is therefore a report of the local experiences and challenges in the six years of application in our local neuro oncology practice.
Methodology
Ethical clearance was obtained from the ethical committee of the Study Centre in December 2021 (Protocol No-WCA/021/2021) to retrospectively review case notes of all patients who had image- guided brain surgeries from January 2016 to December 2021.
The names and the biodata of all consecutive patients who underwent stealth guided diagnostic biopsy or resection/debulking of brain tumors were extracted from the brain tumor registry of the theatre log book. This list was presented to the records office of the administration directorate with an accompanying memo requesting for all case notes to be retrieved. Impeccable records keeping culture of the study center ensured that 100% case notes retrieval was achieved. The study period was six years (January 2016-December 2021). Main study parameters were the key pillars of surgical process including Biodata, Clinical presentation, neuroimaging diagnosis, operation performed (stealth guided diagnostic biopsy or stealth guided resection/tumor debulking), histological diagnosis, adjuvant treatments. Data were analyzed using simple descriptive statistics, and results were presented accordingly.
The basic procedure for neuro-navigation involved 1-Establishment of physical coordinates which can be conventionally frame-based using stereotactic frames or frameless using fiducial markers or surface landmarks. 2-Establishment of imaging coordinates with any or combination of the following imaging modalities – MRI, CT, PET, single-photon emission CT, functional MRI etc. 3-Registration of the imaging coordinates on the computerized systems of the navigation machine including image fusion in some cases that required multiple imaging modalities to achieve better target lesion definition. 4-Co-registration of the imaging coordinates with the actual physical anatomical space, which forms the backbone of neuro-navigation precision. 5-Surgical planning to define surgical entry points, surgical corridor and trajectory incorporating a sound knowledge of neuroanatomy and surgical targets. 6-Navigation, which continues throughout the surgical process. It is important to note that a competent neurosurgeon with a sound understanding of the structure and function of the human cerebrum was always involved. Aside navigation assisted access, the rest of the surgical procedure including microsurgical dissection was as per standard brain tumor surgery protocols and was uniform in all the cases as per the competence of the neurosurgeon. Similarly initial and subsequent post op care was as per standard protocols.
It is also important to note that excluded from this study were other brain tumor operations which did not require the of neuro- navigation technology such as pituitary surgeries. Also excluded were neuro-navigation assisted operations for aspiration and or biopsy of parasitic cysts, abscesses etc.
Various challenges, which were encountered during the surgical navigation processes, were all noted and discussed in this paper. Procurement, installation and maintenance of the necessary high- tech equipment in a practice environment constantly under threat of power supply instability, economic instability, abysmal health insurance policy in the face of high cost of neurosurgical care, paucity of qualified biomedical engineers, inconsiderate duties for essential medical and surgical consumables supply chains, increasing human capital flight (brain drain). Our initial experience and challenges with the first thirty cases of neuro-navigation assisted brain operations in general were already published three years ago but it becomes expedient for an update in the present report which focuses more on neuro-navigation assisted brain tumor operations [9].
Results
There was a total of 111 cases during this study period, 70 males (63%) and 41 females, (M: F = 1.7:1). Age ranges from 8 months to 80 years. Mean 41.9 years, and SD of 20.1 years as detailed in Table 1 age distribution. The commonest presentation was headache 93/111 (84%), Vomiting 39/111 (35%), Seizures 30/111 (27%), Hemiparesis 27/111 (24%), Visual disturbances 21/111 (19%). Others were dysphasia, hearing loss, dysphagia, impairment of consciousness, cognitive decline, loss of balance etc. Pre-op diagnosis were intra axial tumors 70/111 (63%) and extra axial tumors 41/111 (36%). Stealth Guided diagnostic biopsy was the initial step in 68/111 (61%) and it achieved a target precision of 100%. It is important to note that a subset from this latter group proceeded to have additional tumor debulking following an initial diagnostic biopsy especially when the histology returns gliomas. Most of the patients (102=91.9%) underwent neuro-navigation guided tumor resection or maximal safe debulking. They were all the extrinsic tumors -meningiomas (41), metastatic brain tumors (15), all the previously biopsy diagnosed anaplastic astrocytoma (28), six out of the ten biopsy diagnosed GBM, all the pilocytic astrocytoma (4), two out of the six biopsy diagnosed WHO grade II low grade gliomas (LGG), two cases each of schwannomas and medulloblastoma and one case each of ependymoma and oligodendrogliomas. Most of the gliomas were grade III (28/50=56%) followed by glioblastoma multiforme grade IV (10/50=20%), Low-grade glioma grade II (6/50=12%) and pilocytic astrocytoma grade 1 (4/50=8%). Choroid plexus papilloma, Ependymoma and Oligodendroglioma contributed one each (2%). Most of the meningiomas were psamommatous and meningothelial WHO Grade I&II (37/41=90%) but a few 3/41 (7.3%) were anaplastic and one was angiomatous/hemangiopericytoma. The commonest metastatic brain tumor was lung cancer (5/15=33%) closely followed by breast cancer (4/15=26.6%) and prostate cancer (2/15=13.3%). Renal, thyroid, endometrial cancer and multiple myeloma contributed one each. Concerning extent of resection, a gross total resection was achieved in 85 out of the 102 cases (83.3%) who had stealth guided tumor resection or maximal safe debulking surgery. Adjuvant treatments recommended were clinical and radiological surveillance, radio oncology referral etc.
Discussion
All age groups were involved in this brain tumor surgery series. Although some brain tumors are known to be commoner in certain age groups, such delineation was not the focus of this study. Similarly, more males (63%) were observed in this study. However, irrespective of age and sex, brain tumors shared some common symptoms including headache, vomiting, seizures, cognitive decline, and impairment of consciousness.
In a previous study elsewhere [10], headache was also the leading symptom but at a higher rate of 93.33% compared to 84.5% in this series. Common in both studies were other features of raised intracranial pressure due to the presence of intracranial space occupying lesion such as nausea, vomiting, cognitive decline and impairment of consciousness. Other features were either lateralizing or directly related to the functional anatomical location of the tumor such as hemiparesis in tumors close to the motor strip of the posterior frontal lobe, aphasias related to tumors close to the speech areas of the dominant hemisphere. Of particular note is seizures observed mostly in supratentorial tumors. It was observed in 27% of cases in this study but in another report, it was deposited that one third of patients diagnosed with a brain tumor will develop seizures during the clinical course if they did not initially present with seizures [11]
Relevant neuroimaging (CT, MRI) facilitated the diagnosis, tissue definition and accurate anatomical situation of these tumors. This facilitated pre-operative consent discussions and surgical planning towards neuro-navigation assisted diagnostic biopsy or tumor resection or debulking.
Neuro-navigation guided diagnostic biopsy was performed in 68/111 (61%) with a target precision of 100% similar to the previous reports from same center 4. This underscores the whole essence of neuro-navigation where precision targeting must not be compromised and with minimal associated collateral damages. It is particularly suited for deep-seated tumors especially in the brain stem, thalamus and basal ganglia axis. These locations are usually not easily accessible to surgical procedures. It is also extremely useful in other intrinsic tumors situated in different lobes of the brain where an initial minimally invasive diagnostic procedure remains invaluable subsequent evidence-based management. It is instructive to note that from this study, subset from this diagnostic biopsy group proceeded to have additional tumor resection/ debulking surgery following a histologic diagnosis of gliomas especially anaplastic and glioblastoma multiforme (GBM) in a good performance status. Following an initial diagnostic biopsy, with high degree of confidence, all of the anaplastic gliomas proceeded to stealth guided tumor resection or maximal safe debulking surgery, six out of the ten GBM who were in a good performance status also had surgery as above. Only two out of the six LGG who were profoundly symptomatic had surgery as above and the rest were referred to radio-oncology adjuvants because the mere location was risky and surgically unattractive. These were the ones situated deep on the thalamus, basal ganglia and brain stem (pons). The only additional surgery they had was VP shunt to divert the CSF accumulation due to obstructive hydrocephalus consequent upon the tumor location. For low-grade gliomas, there is evidence that early radiotherapy prolonged progression-free survival (PFS) and disease specific survival [12]. However, the attendant risks and side effects of such exposure, including cognitive decline consequent on radiation-induced leukoencephalopathy was a major counselling issue to guide decision-making process [13]. For the rest gliomas cytoreductive surgery followed by external beam radiation has become the standard treatment for newly diagnosed high-grade gliomas against which other treatments are compared [14]. However, there must be unequivocal histological evidence before any such treatment is offered any patient and enough information must be provided to guarantee informed decisions. This is because there is substantial evidence that some CNS mass lesions, including lymphomas could mimic astrocytoma radiologically and stereotactic biopsy targeted at enhancing rim is performed to resolve any diagnostic dilemma [15].
The role of neuro-navigation assistance in the four cases pilocytic astrocytoma surgery was mainly limited to initial direct access to the often-cystic lesion. It is important to note that a remarkable loss of navigation accuracy was expected when the cystic component is drained inadvertently during the process of microdissection. Since up to 50% of pilocytic astrocytoma have an active mural nodule [16]. Which is the usual target for surgical extirpation, this must be achieved by relying more on microdissection expertise since the navigation accuracy was already compromised by brain shift associated by the cyst decompression. It has been a known fact that the surest way of preventing recurrence of pilocytic astrocytoma is establish a complete gross total resection. This position was corroborated in the practice guideline for adjuvant treatments in pilocytic astrocytoma as provided by Austin and Alvord [17]. There were a high 5- and 10-year survival rates. Therefore, the high complication rate of adjuvant radiation therapy in face of slow growth rate of truly pilocytic astrocytoma, does not justify such treatments for pilocytic astrocytoma, rather clinical and radiological surveillance is the practice. Same applies to the Choroid plexus papilloma. However, the medulloblastoma is known to be an advanced stage IV disease right from diagnosis and for the index case, which was standard, risk on stratification, radio oncology referral was the way forward.
The meningiomas constituted 41/111 (36%) of the brain tumors on this study which is slightly lower (44.4%) compared to a previous report from same center [4]. Irrespective of the location (convexity, skull base etc.) all of the (n=41) underwent neuro- navigation assisted resection or maximal safe debulking. The key role of this technology here is appropriate placement of scalp incision, bone flap removal and durotomy to ensure a direct access to the tumor and minimal contact with the surrounding normal brain and other eloquent and neurovascular structures. It also provided a continuous visual near real time feedback on the depth and extent of resection during the microsurgical procedures. Consequently, the collateral morbidities associated with the operations were reduced to the barest minimums. Although complete resection was not possible in some cases either due to close romance with vital neurovascular structures or torrential intraoperative primary hemorrhage, neuro-navigation played a vital role in defining borders for overall safety. This has been corroborated by Schul et al. who confirmed in their study that neuro- navigation was extremely helpful in providing access to these tumors, delineating the margins, identifying the vascular and osseous relations and estimating the extent of resection [18]. Patients recovered quicker post op to proceed to other stages of adjuvant treatments when necessary.
Metastatic brain tumors constituted up to 13% in this study. In a previous and similar report from this center it was 10% [4]. Therefore, there is a marginal increase either because the numbers are larger in this report or because the incidence is increasing. However, it is probably within global prevalence of brain metastases in patients with cancer (8.5%–9.6%) [19]. In this study the most common primary tumors responsible for brain metastases remains lung cancer (5/15=33%) closely followed by breast cancer (4/15=26.6%) and prostate cancer (2/15=13.3%). Renal, thyroid, endometrial cancer and multiple myeloma were also reported. The prominent position of lungs, breast cancers in metastatic brain tumors have been reported previously where lung cancer contributed (19.9%), melanoma (6.9%), renal cancer (6.5%),breast cancer (5.1%) and colorectal cancer (1.8%) [19]. It has also been predicted and rightfully so that this prevalence will likely increase because of increasing length of survival of cancer patient, enhanced ability to diagnose CNS tumors due to availability of neuroimaging (CT/MRI), relative inability of chemotherapeutic agents to cross the blood-brain barrier well, thus providing a safe haven for tumor cells within the brain [20]. Brain metastasis are often well circumscribed and situated on the grey-white matter junction with a lot of surrounding white matter oedema. MRI is more sensitive than CT scan because it detects multiple metastases in in up to 20% of patients who appear to have a solitary lesion on CT scan [21]. It has been well established that brain metastasis is a stage IV disease and an advanced indicator of poor prognosis and nearly always determines a fatal outcome in patients with solid cancers [22]. Therefore, a minimally invasive neuro-navigation guided surgical procedure is most desirable in these circumstances, which marks the beginning of management of end of life. In addition, this forms an integral part of the pre-operative consent discussions. The role of surgical excision in these cases have long been established since the first evidence-based compendium for the treatment of patients with brain metastases published as a level 1 recommendation for surgical resection combined with radiation therapy to prolong life in relatively young patients with good functional status and a newly diagnosed solitary brain metastasis [21]. In addition, this was the guideline followed for all these patients. The role of the neuro-navigation guided procedures was to provide a minimal postoperative morbidity, which ensures preservation of patient dignity and functional independence as long as possible while travelling the inevitable route to end of life. In other words, the surgical procedure must not provide an additional burden on an already precarious situation. It was reassuring that this was achieved in all these cases to justify the insistence on the use of the neuro-navigation technology.
The reasons for incomplete resection in 17/102 (16.6%) varied from proximity to eloquence, neurovascular structures, primary hemorrhage of concern etc. Necessary adjuvant treatments including simple clinical and radiological surveillance was recommended for all the completely resected and WHO grade I and II tumors. For the higher-grade tumors even when completely resected and of course all incompletely resected tumors, radio oncology referral was perfected with of course clinical follow up.
Challenges of Neuro-navigation
Brain shift and Error Margin
The concept of brain shift is an intrinsic limitation of neuro- navigation because of sole reliance on preoperatively co- registration of acquired imaging co-ordinates and anatomical coordinates. There is no chance of intraoperative real-time image updating. The main argument against neuro-navigation has been the brain shift phenomenon, which inevitably happens from head positioning and a lot more following drainage of a large cyst, drainage of CSF, and substantial debulking of tumor. There exist also errors related to registration process [22].
In our center, we have adopted strict adherence to the registration processes such that we work within the acceptable error margin of less than 5mm already in-built in the Medtronic Stealth Treon Plus neuro-navigation system. Other navigation adjuncts including real time ultrasound and intraoperative MRI imaging are already in use in centers who can afford them [2].
Fiducial Markers and Neuroimaging Equipment
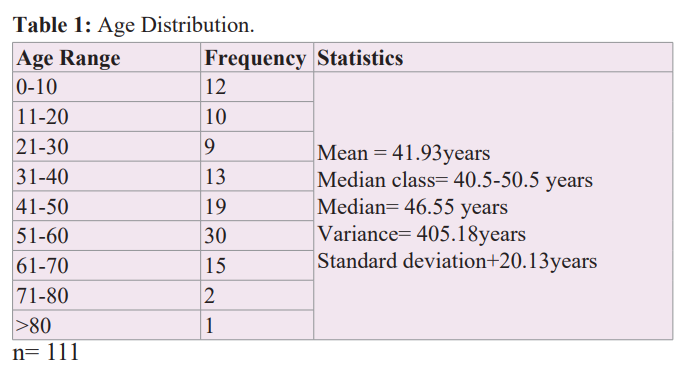
Interruption of the supply chain and premature dislodgement of the fiducial buttons constituted a challenge of concern. A fiducial marker is an object placed in the field of view of an imaging system, which appears on the image produced and to be used as a reference point. One key feature of a good fiducial marker is visibility and discrimination from surrounding tissue. In neuro-navigation it is mainly used to establish imaging coordinates, anatomical coordinates and plays a central role in the all-important process of co-registration. The ideal fiducial marker for MRI neuroimaging is a sticky button affixed to specified anatomical landmarks on the patient’s head, namely nasion, forehead, vertex, inion in the midline and lateral canthus, front of tragus, mastoid bilaterally and making a total of ten points (Figure 1). In the absence of fiducial markers, the only alternative to effect registration and navigation is to rely on surface and anatomical markers provided the necessary software is installed and activated on the Stealth machine and adequate training conducted. The difficulties experienced in regular supply of the traditional fiducial buttons were already highlighted in the previous report (4). In the subsequent years, these difficulties did not only persist, but rather worsened. In addition, the previously reported locally sourced alternative (1 cm × 1 cm blocks of pencil eraser affixed to the patients' head with sticky gum) which lit up brilliantly on CT scan brilliantly but failed to do so on the MRI, continued to be used in our navigation procedures (Figure 2).
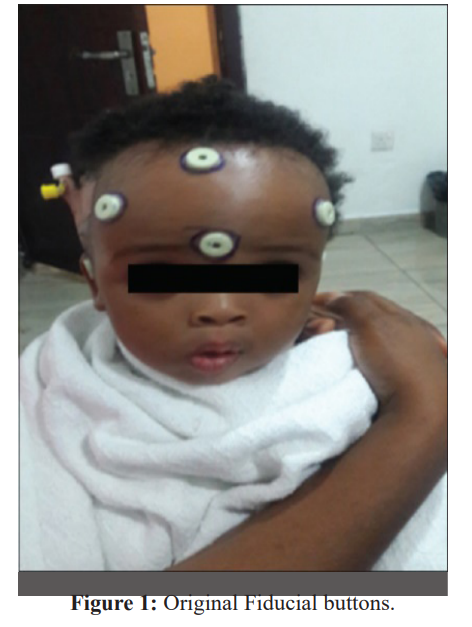
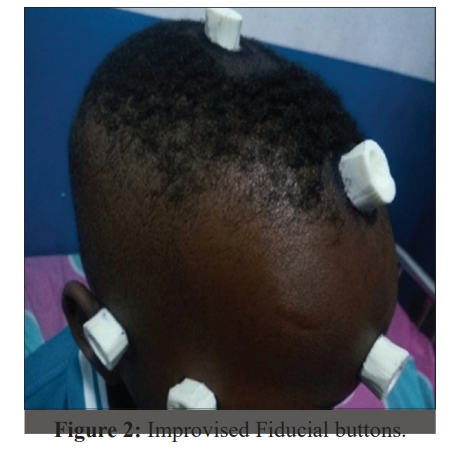
The initial difficulty of transporting patients with fiducial buttons on their head to other centers for scan and the associated risk of premature dislodgement had been completely circumvented by the availability and maintenance of constant imaging services especially the CT scan. Although CT images are traditionally not as good in brain tissue and lesion resolution in comparison to MRI, they were reasonably acceptable in establishing our imaging coordinates for accurate navigation as evidenced by our recorded 100% navigation precision. Premature dislodgement of fiducial markers is a common event recorded in the business of neuro-navigation. It can occur: 1- During transport to and from the radiology unit. 2- In the wards awaiting onwards movement to the operating room while undergoing last minute nursing and pre- operative procedures. 3- In the operating room while undergoing head manipulations during induction of anesthesia and during head fixation on Mayfield clamps. Careful placement of the fiducial buttons, gentle movement of the patients and utmost caution in head handling during anesthesia and application of head fixation clamps until the registration processes are completed are some of the strategies, we have adopted in reducing this challenge.
With neuro-navigation assistance, a wider spectrum of brain tumors were more confidently operated, evidence based histological diagnoses established and adjuvant treatments were more competently deployed in line with a current practice standards for neuro-oncology services. But this success was at a reasonably high cost of adopting measures to overcome some inevitable challenges [23].
Declaration of Patients Consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
References
- Leksell A stereotactic apparatus for intracranial surgery. Acta Chir Scand. 1949; 99: 229-233.
- Archip N, Clatz O, Whalen S, et al. non-rigid alignment of pre-operative MRI, fMRI, and DT-MRI with intra-operative MRI for enhanced visualization and navigation in image- guided neurosurgery. Neuroimage. 2007; 35: 609-624.
- Mirota DJ, Wang H, Taylor RH, et al. A system for video- based navigation for endoscopic endonasal skull base IEEE Trans Med Imaging. 2012; 31: 963-976.
- Charles U, Anthony A, Evaristus N, et al. Computer- assisted brain surgery (neuronavigation) in Abuja, North Central Nigeria: A 3-year retrospective review and practical challenges. Niger Postgrad Med J. 2022; 26: 174-81.
- Khoshnevisan A, Allahabadi NS. Neuronavigation: principles, clinical applications and potential pitfalls. Iran J Psychiatry. 2012;7: 97-103.
- Mitsui T, Fujii M, Tsuzaka M, et al. Skin shift and its effect on navigation accuracy in image-guided Radiol Phys Technol. 2011; 4: 37-42.
- Moiyadi A, Shetty P. Objective assessment of utility of intraoperative ultrasound in resection of central nervous system tumors: A cost-effective tool for intraoperative navigation in neurosurgery. J Neurosci Rural Pract. 2011; 2: 4-7.
- Watanabe Y, Hayashi Y, Fujii M, et al. Development of automatic navigation measuring system using template- matching software in image guided neurosurgery] Nihon Hoshasen Gijutsu Gakkai Zasshi. 2010; 66: 131-136.
- Charles U, Anthony A, Evaristus N, et al. Computer- assisted brain surgery (neuronavigation) in Abuja, North Central Nigeria: A 3-year retrospective review and practical challenges. Niger Postgrad Med J. 2022; 26:174-81.
- Datta PK, Sutradhar SR, khan MN, et Clinical Pattern of Intra-cranial space occupying lesion in Tertiary Level Hospital. Journal of Dhaka Medical College. 2020; 28: 17-22.
- Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. 2000; 54: 1886-1893.
- Hanzély Z, Polgár C, Fodor J, et Role of early radiotherapy in the treatment of supratentorial WHO grade II astrocytomas: Long- term results of 97 patients. J Neurooncol. 2003; 63: 305-312.
- DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989; 39: 789-96.
- Stupp R, Mason WP, van den Bent MJ, et Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352: 987-96.
- Greene GM, Hitchon PW, Schelper RL, et Diagnostic yield in CT-guided stereotactic biopsy of gliomas. J Neurosurg. 1989; 71: 494-497.
- Gol A. Cerebellar astrocytomas in children. Am J Dis Child 1963; 106: 21-24.
- Austin EJ, Alvord EC Recurrences of cerebellar astrocytomas:A violation of Collins' law. J Neurosurg. 1988; 68: 41-47.
- Schul C, Wassmann H, Skopp GB, et Surgical management of intraosseous skull base tumors with aid of operating arm system. Comput Aided Surg. 1998; 3: 312-319.
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance J Clin Oncol. 2004; 22: 2865-2872.
- Mintz AP, Cairncross JG. Treatment of a single brain metastasis: The role of radiation following surgical JAMA. 1998; 280: 1527-1529.
- Owonikoko TK, Arbiser J, Zelnak A, et Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014; 11: 203-222.
- Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96: 33-43.
- Verploegh IS, Volovici V, Haitsma IK, et al. Contemporary frameless intracranial biopsy techniques: Might variation in safety and efficacy be expected? Acta Neurochir (Wien). 2015; 157: 2011-2016.