Th1 and Th2 Cytokines Pattern among Sickle Cell Disease Patients in Côte d’ivoire
Author'(s): Liliane K. Siransy1*, Chiayé C.A. Yapo-Crézoit2 , Maxime K. Diane3 , Sidonie Goore3 , Saydou Kaboré3, Bettina Koffi-Kabran3 and Seidou Konaté3
1Félix Houphouet Boigny University- Medical Sciences-Immunology -Allergology Department, 1, boulevard del’Université, Cocody, BP V 34 Abidjan-Côte d’Ivoire.
2 Pasteur Institute of Côte d’Ivoire, Biology of Immunity department Cocody, 01 BP 490 Abidjan-Côte d’Ivoire.
3 National Blood Transfusion Center, Therapeutic and Research unit 52, boulevard de Marseille, Zone 3, BP V 15 Abidjan-Côte d’Ivoire.
*Correspondence:
Liliane Kouabla Siransy, Félix Houphouet Boigny UniversityMedical Sciences -Immunology -Allergology department, 1, boulevard de l’Université, Cocody, Abidjan-Côte d’Ivoire, Tel: +22567650761; Fax: +225 21358060; E-mail: lsiransy@gmail.com.
Received: 13 January 2018; Accepted: 16 February 2018
Citation: Liliane K. Siransy, Chiayé C.A. Yapo-Crézoit, Maxime K. Diane, et al. h2 and h2 Cytokines Pattern among Sickle Cell Disease Patients in Côte d’ivoire. Clin Immunol Res. 2018; 2(1): 1-4.
Abstract
Introduction: Sickle cell disease (SCD) is the most prevalent genetic disease worldwide and particularly reaches its highest prevalence in sub-Saharan African countries. In Côte d’Ivoire, the SCD prevalence rate is 12%. Many evidences show that the immune system plays an important role in this inflammatory condition with secreting inflammatory cytokines.
This work attempts to identify the cytokine pattern displayed by Ivorian patients during the course of disease, as Th1 cytokines, as well as Th2 cytokines. Furthermore, this study desires to contribute to identify advantages of chronic transfusion in SCD patients.
Patients and Methods: 49 subjects (4 to 55 years) were prospectively enrolled in the study after an informed consent. The patients were assigned in 2 groups, patients in steady state and patients in crisis. Serums were measured in San Diego Biolegend laboratory by using LEGENDplexTM Human Inflammation Panel assays and ELISA.
Results: Evaluation of serum cytokines in SCD crisis patients revealed an increased level for IL-1β, IL-6 and IL-10 cytokines. The IFN-γ to IL-4 ratio was 1.92 in crisis subjects and 3. 55 in steady state subjects indicating a trend toward a Th2 bias in crisis patients.
Conclusion: The role of cytokines in role in pathogenesis and progression in SCD is well established. However, accurate data are lacking for people with SCD in Sub-Saharan Africa where the disease is endemic. This study reveals a Th2 bias in SCD patients. The reduction in cytokine levels observed in our transfused patients provides an overview of the definite benefit of chronic transfusion.
Keywords
Introduction
Sickle cell disease (SCD) is the most prevalent genetic disease worldwide and particularly reaches its highest prevalence in sub- Saharan African countries. Particularly in areas around the equator, the prevalence varies between 20% and 30% [1,2]. In Côte d’Ivoire, the SCD prevalence rate is 12 % [3]. As such, SCD is one of the greatest public health treat and represent a public health problem. Sickle cell disease (SCD) is in fact a chronic inflammatory disease and the permanently activated immunoinflammatory status exhibit increased levels of proinflammatory cytokines with chronic hemolysis, frequent infections, recurrent clinical and subclinical occlusion of microcirculation and result in tissue injury, chronic organ damage and failure and ultimately, premature death [4,5]. Classically two clinical profiles are well known, sickle cell steady state and sickle cell crisis. The steady state is a period during which the patient feels well but it‘s a precarious situation which can degenerate into the second profile, the vasoocclusive crisis, at any time [6]. The mechanisms by which sickle cell crisis are initiated are complex and multifactorial and involves many factors such as cytokines [6,7]. Many evidences show that both type I and type II cytokines are significantly altered in SCD patients. CD4+ and CD8+ cells are further divided into subsets by their function and pattern of cytokine secretion. Type 1 (h2) cells typically produce IL-2, tumor necrosis factor- β (TNF- β) and interferon α(IFN α), while type 2 (h2) cells produce IL-4, IL-5, IL-6, IL-9 and IL- 10 . A predominance of h2 cytokines results in the induction of vigorous mediated immunity while a predominant h2-biased response results in stronger humoral immunity [8].
While there are published reports of individual cytokines in sickle cell disease patients, few studies are really investigating the relative levels of h2-type and h2-type cytokines in black Africans. This work attempts to identify the cytokine pattern displayed by these patients during the course of disease, as h2 cytokines, such as TNF α, IL-1 β, IL-12p70 and IFNγ, as well as h2 cytokines, such as IL-4, IL-6 and IL-10. Furthermore, this study desires to contribute to identify factors that can help for a better comprehension of the physiopathology and therapeutics of the disease.
Patients and Methods
Study population
This is a prospective study with 49 SCD patients from 4 to 55 years old followed at the transfusion therapeutic unit located in National blood Transfusion Center in Abidjan, Côte d’Ivoire. It was conducted between from October 2016 to February 2017 after approval from the national ethics board and informed consent was obtained from the patients’ parents.
Each subject was prospectively enrolled in the study after an informed consent was obtained. Patients were assigned in 2 groups: patients in steady state (no acute illness, no crisis or infection during the previous 3 months) for the first group and patients admitted for anemia, infection, and crisis for the second group.
Documentation of homozygous or heterogynous sickle cell patients had been determined by hemoglobin electrophoresis on cellulose acetate strips (PH 9.2). The vaso-occlusive crisis (VOC) patients were admitted to the unit and those who were non-symptomatic, steady state sickle cell patients coming at the unit for routine check-ups.
Vaso-occlusive crisis (VOC) was defined as an episode of diffuse acute pain occurring in the abdomen, back, chest, bone, joint, or multiple sites of pain, necessitating hospital admission and or analgesic administration. Clinical investigations were assessed by hematology specialist.
Steady-state SCD control patients were matched with crisis SCD patients according to sex, gender, hemoglobin type, hemoglobin level, body mass index (BMI) and chronic transfusion. All subjects were coming most from Côte d’Ivoire and West African countries (Nigeria, Togo, Ghana, Mali, and Guinea).
Cytokines assays
Blood sample were collected by veinopuncture in EDTA for the determination of the basic hematological indices and for the cytokine assay. Complete blood counts were obtained with an electronic cell counter (Sysmex XN 550 Hematology analyzer). The plasma was separated from the tube sample at 1,000g at 4°C for 10 min, aliquoted and stored at -30°C for cytokine assays. Prior to use, the samples were thawed completely, mixed and centrifuged. Serums were measured by using BioLegend’s LEGENDplexTM Human Inflammation Panel assays which is bead-based immunoassays, using fluorescence encoded beads and The BioLegend LEGEND MAX™ Human IL-4 ELISA Kit. These panels allow simultaneous quantification of many human inflammatory cytokines and chemokines, but we had focused on h2 cytokines (IFN γ, IL-1β, TNF α, IL-12 p70) and h2 cytokines (IL-4, IL-6, IL-10). The assay was performing using ELISA for IL-4 and a filter plate for the others cytokines in Biolegend laboratory in San Diego, US. A minimum of 3000 positive beads for these cytokines was acquired with a cytometer type BD FACSCalibur™. The BioLegend LEGEND MAX™ Human IL-4 ELISA Kit, Sandwich Enzyme- Linked Immunosorbent Assay (ELISA) with a 96-well strip plate that is pre-coated with a capture antibody was used to detect IL-4. All samples were run and analyzed on the same day after being thawed completely. Manufactured supplied controls were used to monitor coefficients of variation, which were <10%. The minimum detectable concentration for h2 and h2 cytokines were respectively IFN γ: 1.5pg/ml, IL-1β: 0.9pg/ml, TNF α: 1.0 pg/ml, IL-12 p70: 0.6pg/ml, IL-4: 0.6 pg/ml, IL-6: 1pg/ml, IL-10: 0.8pg/ml. Data analysis was done using LEGENDplexTM Data Analysis Software when data acquisition is completed.
Statistical analysis
All results are expressed as mean+/- SD. Data were analyzed using the SPSS, version 15, for Windows. Statistical significance was calculated using Student’s unpaired t test, the Mann-Witney U test, Chi-square and Fisher’s exact test. Statistical test results with a P value ≤0.05 were considered to be significant.
Results
Demographic characteristics in the sickle cell patients
Fourty nine patients with a diagnosis of sickle cell disease (SCD), comprising 26 males (53.06%) and 23 females (46.94 %), were recruited.
The mean age for all SCD patients were respectively, 27.82 ±13.77 years (min/max= 4/50 years) for crisis group and 20 ± 13.74 (min/max= 4/55 years) for the steady state group. There was no significant difference in the age range between group 1 and group 2 (p value= 0.09) and the sex ratio was 1 and 1.3 (in favor of men) respectively in group 1 and 2 (Table 1).
in steady state subjects were respectively 7.27 ± 11.30 pg/ml, 2.10± 4.46 pg/ml , 7.05 ± 12.26 pg/ml, 1.30 ± 2.14 pg/ml. There were no significant differences in the 2 groups (Table 2).
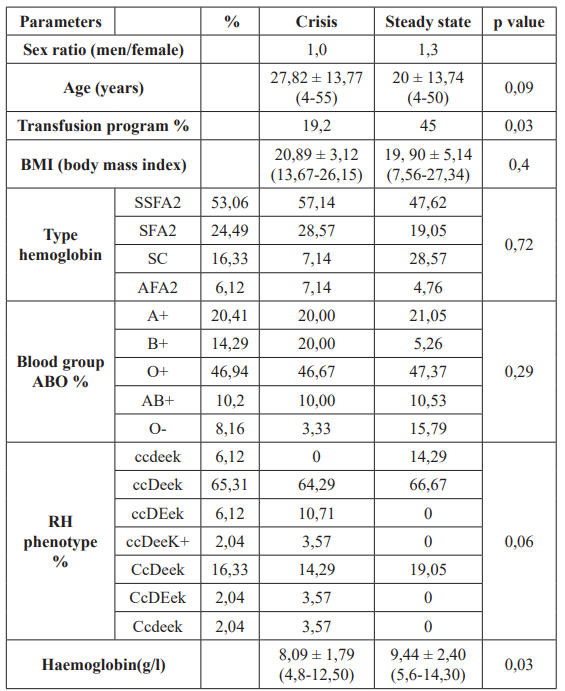
Table 1: Demographics characteristics of SCD patients in Côte d'Ivoire.
Of the SCD patients, 21 (42.86 %) were in steady state and represented our stable controls (group 1), while 28 (57.14%) were in crisis (group 2). Regarding hemoglobin type, 24 were HbSS homozygous type (53.06%), 14 were Sβ+ Thal heterozygous type (24.49%), 8 HbSC (16.33%) and 3 βThal homozygous type (6.12%).
The patients were phenotyped in ABO and Rh Kell. In ABO system, the frequency of blood group were as followed: A Rh D (20.41%), B Rh D (14.29%), O Rh D (46.94%), AB Rh D (10.2%),O Rh D negative (8.16%) (Table1). In Rh Kell system, the ccDeeK phenotype was the most prevalent in both two groups (66.67% for group 1 and 64.29% for group 2).
Sickle cell disease was associated with lower level of hemoglobin in steady state and crisis group (respectively 9.44 ± 2.40 and 8.09 ± 1.79 g/l), showing a significant variation (p=0.03).
Plasma levels of cytokines
The median concentrations for h2 and h2 cytokines measured are shown in Table 2.
The data in table 2 show the levels of IFN-γ, TNF a, Il-1β and IL- 12 p70 (h2 cytokines) in the sera of patients with crisis compared to those in steady-state. The mean level of these h2 cytokines in the sera of crisis subjects were respectively 6.64 ± 8.20 pg/ml, 4.05± 9.73 pg/ml, 41.65 ± 183.26 pg/ml, 4.23 ± 9.14 pg/ml while levels
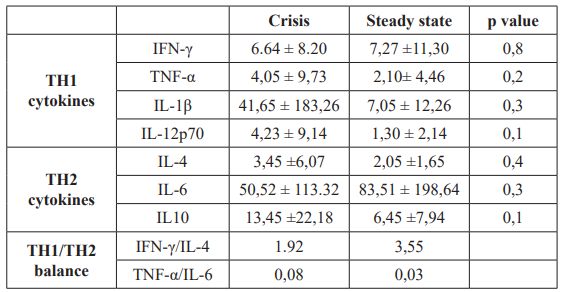
Table 2: Levels of cytokines in SCD patients.
The data in table 2 show also the levels of IL-4, IL-6 and IL-10 (h2 cytokines) in the sera of patients with crisis compared to those in steady-state. The mean level of these h2 cytokines in the sera of crisis subjects were respectively 3.45 ± 6.07 pg/ml , 50.52± 113.32 pg/ml, 13.45 ± 22.18 pg/ml, while levels in steady state subjects were respectively 2.05 ± 1.65 pg/ml, 83.51 ± 198.64 pg/ ml , 6.45 ± 7.94 pg/ml. There were no significant differences in the 2 groups (Table 2).
Chronic transfusion was done in 45% of patients in steady state versus 19.2% in crisis patients (p=0.03). According to their belonging to chronic transfusion program, the mean level of IL1β and IL-6 was lower in patients receiving chronic transfusion. (8.16± 12.78pg/ml versus 33.55 ± 161.78pg/ml for IL1β and 28.15 ± 38.16pg/ml versus 78.35 ± 179.37pg/ml). No significant variation was noted (Table 3).

Table 3: Levels of cytokines in SCD patients according to chronic transfusion program.
h2/h2 balance
We calculated the ratios of the h2 to h2 cytokines in the plasma in view of the fact that the ratios of h2 to h2 cytokines are more relevant and pertinent than the levels of these cytokines alone.
The IFN-γ to IL-4 ratio is 1.92 in crisis subjects and 3. 55 in steady state subjects indicating a trend toward a h2 bias in crisis patients (Table 2). The TNF a to IL-6 ratio is 0.08 in crisis compared to 0.03 in steady state patients and show also a h2 bias in crisis patients.
Discussion
Studies of cellular immunity and humoral factors have yielded conflicting results in sickle cell disease. Furthermore, most reports of the roles and pattern of cytokines have focused mainly on non African patients. In this study, we want to give our contribution on how the immune system plays an important role in SCD in Ivorians patients. Many evidences show that the chronic inflammatory state characterizes this disease, in both crisis and steady state conditions. In addition to an activated endothelium and leukocytosis, the inflammatory phenotype of sickle cell disease is further characterized by rise levels of acute phase proteins and cytokines [9]. Several cell types secrete pro-inflammatory cytokines that contribute to the occurrence of common cyclical events in SCD patients [10].
Plasma levels of some h2 and h2 cytokines were evaluated in SCD steady state and crisis groups in order to estimate the role of these cytokines in SCD and the inflammatory profile of the patients.
Evaluation of serum cytokines in SCD crisis patients in the present study revealed an increased level for IL-1β and IL-10 cytokines in crisis group. IL-6 was higher in steady state group (83.51pg/ml versus 50.52pg/ml) with no significant variation and only IL-6 was higher in this group (Table 2).
In a similar study [11] detectable levels of IL-1β ( crisis: 3.26 pg/ ml; steady state: 3.19 pg/ml) and IL-6 (crisis :14.18 pg/ml, steady state: 6.78 pg/ml) were found in the serum of patients in the steady-state condition and crisis although this was not statistically significant.
On the other hand, other authors showed that the mean serum IL-1β and IL-6 were respectively and significantly increased in the both group steady state (44.36 pg/ml/137.62 pg/ml) and crisis (42.59 pg/ml/175.7 pg/ml) [6]. Thus, contradictory findings are reported by multiples studies and may be due to the low number of SCD patients, the selection criteria and SCD phenotype and genetic polymorphism of the cytokine [11-14].
No significant detectable levels of IFNγ, TNFα, and IL-12p70 were found in the sera of either group of patients although a slight increase was noted for TNFα and IL-12 in crisis group. Others authors show higher levels in both groups [12,13,15].
In our study, detectable levels of IL-12 were observed in crisis without significant difference as shown by Taylor et al. [16]. IL- 12 is composed of p35 and p40 subunits, which, when combined together form the bioactive IL-12p70 [17]. IL-12 is predominantly proinflammatory and prostimulatory cytokines and a major function is the induction of IFN-γ produced by NK cells, T cells, B cells, and even antigen-presenting cells [16]. Recently, the involvement of IL-12, in inflammatory responses in SCA patients has been described and thus appears to be the main cytokine that regulates h2 differentiation [10,17,18]. In addition, IL-12 antagonizes h2 differentiation and the production of IL-4 and is inhibited by IL-10 [10].
Levels of IL-4 are low in steady-state (2.05 ± 1.65pg/ml) and crisis (3.45 ± 6.07pg/ml) SCD patients in this study and did not differ significantly (p=0.4). The role of IL-4 is controversial. While some studies have reported that IL-4 levels increase during VOC, other studies have demonstrated that IL-4 levels are higher during the steady-state [8,19]. An investigation of the h2 cytokine revealed that plasma IL-4 levels were significantly higher among steady- state HbSS patients than HbAA and HbAS individuals and may differ between children in developed countries and children in developing countries [20] due to undernutrition.
In our SCD patients, a trend toward higher IL-10 levels in patients in crisis than in steady state was found with no significant variation. Conflicting reports are reported on the role of IL-10 in SCD patients. Sarray [21] demonstrated that changes in IL-10 serum levels is predicting VOC in SCD patients and his results clearly demonstrated a significant association between reduced IL- 10 levels and the development of VOC and its severity. Patients undergoing Hydroxyurea therapy had high levels of IL-10 [10]. IL- 10 has a protective effect against the occurrence of severe malaria in patients with sickle cell trait and confirm the contribution of this cytokine to the regulation cellular iron status [10].
h2-type cytokines activate anti-microbial functions in phagocytes which determine protection against intracellular bacteria and protozoa while h2-type cytokines, activate B cells and induce antibody production, a function that is vital to the neutralisation of microbial toxins and certain viruses. The ratios of h2 to h2 cytokines are more relevant and pertinent than the levels of these cytokines alone. As IFN-γ inhibits the expression of h2 cytokines such as IL- 4, and vice versa, the IFN- γ /IL-4 cytokine ratio is considered to be a simple and direct indicator of h2/h2 balance. The IFNγ to IL-4 ratio in plasma indicates a h2 bias in SCD patients [8,20]. Our observations support the same trend by showing a h2 pattern.
With advances now reducing the side effects of transfusion over the last decade clearly defining the efficacy for decreasing sickle cell morbidity, the indications for transfusion have increased [22,23]. Chronic transfusion lead to a significant improvement in quality of life and is indicated for the prevention of primary and secondary stroke, acute cerebrovascular accidents and severe acute chest syndrome. A way to decrease HbS level without raising hematocrit is to associate phlebotomy and transfusion, which defines exchange transfusion. Patients receive RBC transfusions via simple transfusion, partial exchange transfusion or erythrocytapheresis, every 4-5 weeks, depending on quantitative hemoglobin S concentration. It may be performed manually, exchanging patient’s whole blood with packed red cells, or using a cell separator, with reinfusion of the patient’s plasma [24].
In our study, h2 and h2 cytokines levels were lower in chronic transfused patients compared to non transfused SCD patients but the variation was not significant (Table 3). Leukocyte-reduced units have beneficial effects in reducing alloimmunization, transfusion reactions, platelet refractoriness, and infection transmission [25] but in our area, they are little used because of lack of materials. The low levels of cytokines in our SCD patients should offers insight into the mechanism of the protective effect of transfusion [26].
Patients who are compliant with blood transfusion are rarely hospitalized for painful crises [22]. Kalechman demonstrate in his study that 2 weeks after blood transfusion, a sharp decrease was observed in the generation of some cytokines. A decrease of about 70% was observed in interleukin-2, tumor necrosis factor, and gamma-interferon secretion. Actually, apheresis can remove pathogens and mediators that contribute to pathogenic inflammatory responses in diseases and should be introduced in local management of our patients [27].
Our study has some limitations. First, the results are based on data from small number of samples and a multi-center study was required to be performed to validate the results. Second, a small increase in circulating cytokine levels may reflect a significant increase in cytokine concentration at the tissue level. As Tam et al. [28], the systemic measures in our study may underestimate the concentrations at a local level. Therefore our results should also be interpreted with finesse and further studies should take in consideration both local and systemic aspects. Third, In Africa and particularly, in Ivory Coast, undernutrition is prevalent. 15-20% of the population suffers from Under nutrition. In general, this figure is associated with poor immune function and is consequently regarded as the most common cause of immunodeficiency worldwide [29]. This may have strong impact on cytokine secretion.
Conclusion
The present study demonstrates a h2 driven immune response SCD patients. Understanding the roles of different cytokines in the pathology of SCD is essential for the development of effective therapies for this disease. The reduction in cytokine levels observed in our transfused patients provides an overview of the definite benefit of chronic transfusion but apheresis in this area is still little used and even absent in transfusion protocols. Research including more subjects and more research should be carried out in the future for further exploring and reducing morbidity and mortality.
Acknowledgment
We appreciate the hands on training, the excellent technical assistance and the donation of reagents by Shi Shaoquan and Sun Biggang and in Biolegend, in San Diego. The authors thank Kady Kassogue for help with obtaining patient consents, patient transfusion data and blood sample and dr Aholi for his help in the statistical analysis of the data.
References
- Diallo D, Tchernia Sickle cell disease in Africa. Curr Opin Hematol. mars 2002; 9: 111-116.
- Williams TN. Sickle Cell Disease in Sub-Saharan Africa. Hematol Oncol Clin North Am 2016; 30: 343-358.
- Le Gallais D, Lonsdorfer J, Fabritius H, et al. Prevalence of the sickle cell trait among students in a physical education college in Côte-d’Ivoire. Nouv Rev Fr Hematol. 1989; 31: 409-412.
- Akohoue SA, Shankar S, Milne GL, et Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatr Res. Févr. 2007; 61: 233-238.
- Bandeira ICJ, Rocha LBS, Barbosa MC, et Chronic inflammatory state in sickle cell anemia patients is associated with HBBS haplotype. Cytokine. Févr. 2014; 65: 217-221.
- Pathare A, Kindi SA, Daar S, et al. Cytokines in sickle cell Hematol Amst Neth. Oct. 2003; 8: 329-337.
- Makis AC, Hatzimichael EC, Bourantas The role of cytokines in sickle cell disease. Ann Hematol. Août. 2000; 79: 407-413.
- Raghupathy R, Haider MZ, Azizieh F, et al. h2 and h2 cytokine profiles in sickle cell disease. Acta Haematol. 2000; 103: 197-202.
- Olenscki Gilli SC, Pericole FV, Benites BD, et al. Cytokine polymorphisms in sickle cell disease and the relationship with cytokine Exp Hematol. Juill. 2016; 44: 583-589.
- Pitanga TN, Vilas-Boas W, Cerqueira BAV, et al. Cytokine profiles in sickle cell anemia: Pathways to be unraveled. Adv Biosci Biotechnol. 3 juill. 2013; 04: 6.
- Keikhaei B, Mohseni AR, Norouzirad R, et al. Altered levels of pro inflammatory cytokines in sickle cell disease patients during vaso occlusive crises and the steady state condition. Eur Cytokine Mars. 2013; 24: 45-52.
- Pathare A, Al Kindi S, Alnaqdy AA, et Cytokine profile of sickle cell disease in Oman. Am J Hematol. 2004; 77: 323- 328.
- Sarray S, Saleh LR, Lisa Saldanha F, et Serum IL-6, IL-10, and TNFα levels in pediatric sickle cell disease patients during vasoocclusive crisis and steady state condition. Cytokine. Mars. 2015; 72: 43-47.
- Vicari P, Adegoke SA, Mazzotti DR, et Interleukin-1β and interleukin-6 gene polymorphisms are associated with manifestations of sickle cell anemia. Blood Cells Mol Dis. Mars. 2015; 54: 244-249.
- Ojo OT, Shokunbi WA, Ibijola AA, et al. Serum Levels of Gamma-interferon and Interleukin-4 in Homozygous Sickle Cell Anaemia Patients. Int Blood Res avr. 2016; 5: 1-7.
- Taylor SC, Shacks SJ, Qu Z. In vivo production of type 1 cytokines in healthy sickle cell disease patients. J Natl Med nov. 1999; 91: 619-624.
- Gee K, Guzzo C, Che Mat NF, et The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. Mars. 2009; 8: 40- 52.
- Vignali DAA, Kuchroo IL-12 Family Cytokines:Immunological Playmakers. Nat Immunol. 2012; 13: 722-728.
- Musa BOP, Onyemelukwe GC, Hambolu JO, et Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso occlusive crisis. Clin Vaccine Immunol CVI. Avr. 2010; 17: 602-608.
- Knight-Madden J, Vergani D, Patey R, et Cytokine levels and profiles in children related to sickle cell disease and asthma status. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. 2012; 32: 1-5.
- Sarray S, Almawi Contribution of Reduced Interleukin-10 Levels to the Pathogenesis of Osteomyelitis in Children with Sickle Cell Disease. Clin Vaccine Immunol CVI. 2015; 22: 1020-1024.
- Master S, Ong M, Mansour Blood Transfusion in Adult Patients with Sickle Cell Disease: Incidence of Alloimmunization. Blood. 2 déc. 2016; 128: 4859.
- Sakhalkar VS, Rao SP, Weedon J, et Elevated plasma sVCAM-1 levels in children with sickle cell disease: Impact of chronic transfusion therapy. Am J Hematol. 2004; 76: 57-60.
- Montalembert M Échanges érythrocytaires chez les patients drépanocytaires. Hématologie. 2007; 13: 243-249.
- Vichinsky Current issues with blood transfusions in sickle cell disease. Semin Hematol. 2001; 38: 14-22.
- Kalechman Y, Gafter U, Sobelman D, et al. The effect of a single whole blood transfusion on cytokine secretion. J Clin Mars. 1990; 10: 99-105.
- Sloan SR, Andrzejewski C, Aqui NA, et Role of therapeutic apheresis in infectious and inflammatory diseases: Current knowledge and unanswered questions. J Clin Apheresis. 2015; 30: 259-264.
- Tam CS, Garnett SP, Cowell CT, et al. IL-6, IL-8 and IL-10 levels in healthy weight and overweight children. Horm Res 2010; 73: 128-134.
- van Wesenbeeck CF, Keyzer MA, Nubé M. Estimation of undernutrition and mean calorie intake in Africa: methodology, findings and Int J Health Geogr. 2009; 8: 37.