The Development of LPPC in PAS in Blood Transfusion Centre
Author'(s): Jongkol Akahat1, Thipaporn Jaroonsirimaneekul1, Nuanchan Mungkhunkhamchaw1 and Kutcharin Phunikhom1,2*
1Blood Transfusion Centre, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
2Departments of Pharmacology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
*Correspondence:
Kutcharin Phunikhom, Departments of Pharmacology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand,E-mail: kutcha_s@kku.ac.th.
Received: 15 June 2018; Accepted: 24 July 2017
Citation: Akahat J, Jaroonsirimaneekul T, Mungkhunkhamchaw N. The Development of LPPC in PAS in Blood Transfusion Centre. Cancer Sci Res. 2018; 1(3); 1-4.
Abstract
Background and Objective: Transfusion support in cancer patients is vital for their survival. Platelets, in particular, are necessary to prevent serious bleeding. Platelet additive solutions (PAS) are crystalloid nutrient media used in place of plasma for platelet storage. They replace 60-70% of plasma in platelet components. So the amount of storage plasma can be decreased. Platelet stored in PAS have been demonstrated to have a lower risk for allergic transfusion reactions and appeared to have equivalent clinical efficacy for controlling bleeding, compared to platelets stored in 100% plasma. We try to bring PAS to replace plasma in making leukocyte poor platelet concentrates (LPPC) compared with conventional methods that use plasma, 1 bag of total buffy coat 4 units.
Objective: The objective of this study was to compare LPPC in PAS and the traditional LPPC in order to prepare LPPC in PAS in our routine work.
Methods: PAS and plasma using a ratio of 65:35 in accordance with the standard reference. Then LPPC in PAS were measured for the volume, content of platelet concentrates, white blood cell contamination and the titer of anti-A and anti-B compared to traditional methods.
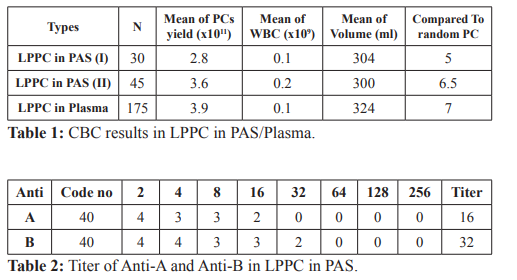
Results: LPPC in PAS in the first trial had volumes 304 ml, content of platelet concentrates 2.8X1011 cells/unit and had 0.1X109 white blood cells contamination. LPPC from traditional methods had volumes 324 ml, contents of platelet concentrates 3.9X1011 cells/unit and had 0.1 X109 white blood cells contamination. The platelet yields showed significant different (p>0.1). In the second trial, LPPC in PAS had volumes 300 ml, content of platelet concentrates 3.6X1011 cells/unit and had 0.2X109 white blood cells contamination. It’s not significant different from the traditional method (p<0.1). The titer of anti-A and anti-B in LPPC in PAS is less than or equal to 64, all of which are classified as low titer, but LPPC from the traditional way with a titer of anti-A and anti-B over 64 about 20%.
Conclusion: All of LPPC in PAS are classified as low titer, which led to the patient at any group. This would make the platelet concentrates inventory more flexible. Content of platelet concentrates and white blood cells contamination from LPPC in PAS provides reached the recommended quality of Council of Europe (EU) and National Blood Centre, Thai Red Cross Society (TRC).
Keywords
Introduction
The transfusion of platelet concentrates prepared from allogeneic single or pooled donations is a standard procedure in transfusion radiotherapy [1]. Platelet concentrates (PCs) are specialized blood cells that play central roles in physiologic and pathologic processes of hemostasis, wound healing, host defense, thrombosis, inflammation, and tumor metastasis. Activation of platelets is crucial for platelet function that includes a complex interplay of adhesion, signaling molecules, and release of bioactive factors. Transfusion of platelet concentrates is an important treatment component for thrombocytopenia and bleeding. The usual adult dose is 4-6 units of random donor platelets. The separation of platelets from whole blood is based on the differential densities of various cellular components when blood is subjected to variable centrifugation forces. PCs for transfusion can be prepared by three different methods including (i) platelet-rich plasma-platelet concentrates (PRP PC), (ii) buffy coat-platelet concentrates (BC- PC), and (iii) apheresis-platelet concentrates (apheresis-PC).
Platelet additive solutions (PAS) have been used to store platelets since the 1980s [2,3]. PAS storage of pooled buffy coat prepared platelet concentrates (PC) have long been used in Europe [4]. The advantages of using PAS for platelet storage are many including more plasma to meet patient needs or to fractionate into plasma-based products, reduced red cell hemolysis from ABO incompatible plasma and reduce other adverse effects related to plasma transfusion [5-7]. Reduction in plasma volume with anticipated benefits in reduced allergic reactions, and possibly transfusion-related acute lung injury (TRALI) [8].
PAS compose of citrate, acetate, phosphate, magnesium, potassium and gluconate. Each type of PAS, these components will be different. PAS has been used as a platelet storage medium in apheresis platelets and buffy coat-derived platelets. It is not only used, but used in combination with plasma. The propotion of plasma range from 20 to 50% and PAS 50 to 80%.
The advantages of PAS are improve the efficiency of platelet collections: additional volume for more collection, maximize the capability to collect multiple blood components, facilitate pathogen inactivation, increase storage time to 7 days with bacterial detection test and due to decrease in the titer of ABO agglutinins, platelets in PAS do not require ABO compatibility between donor plasma and recipient cells or use as universal platelet.
Buffy coat pooling, usually 4-6 BCs are connected to a pooling system / SCD connected to each other, together with 1 unit of PAS or plasma (sterile docking for storage). BC bags washing with PAS/plasma and then into the bottom bag. Soft spin centrifugation of BC pool and transfer upper PC into storage bag with manual press or with an automated separation device, leaving BC-RBC layer into pooling bag. PC could be sampled, stored or filtered during transfer or with an automated instrument (eg.TACSI).
The advantages of pooled buffy coat platelet are higher yield of platelet, reduce standard dose from 6 to 4 units per pool, reduce donor exposure, lower residual white blood cell and increase plasma recovery.
Objective
The objective of this study was to compare LPPC in PAS and the traditional LPPC in order to prepare LPPC in PAS in our routine work.
Methods
Whole blood (WB; 450+10% ml) was high-speed at 3,960 rpm, 22°C, 10 minutes centrifuged (Heraeus Cryofuge 6000i, 8500i) before separation by semi-automated system. Buffy coat was separate for 30-35 ml packed. Platelet concentrate (PC) was prepared by pooling four iso-group buffy coat (BC) units, resuspened with PAS or plasma on the next day. The pooled was low-speed centrifugation at 2,300 rpm, 22°C for 4 minutes and transferring the supernatant (LPPC) to a 5-days storage bag (PL 2410 plastic storage container 1,000 ml). Both LPPC and LPPC in PAS were sampled and stored in a flat agitator at 20-24°C for up to 5 days after collection. Weights, platelet yields were measured and volumes were calculated based on specific gravity. For counting residual leukocytes were performed by automate; Mythic 22 hematology analyzer. The titer detection used two-fold dilution by standard tube method.
The First Trial
We selected the four iso-groups that passed blood processing such as ABO blood group, infectious markers and antibody screening detection. In the traditional method used 1 bag of plasma, it’s the one bag of four iso-group, but the new method used only 100 ml of plasma, then connected them with sterile connecting device. We used plasma, washed buffy coat from the top bag to the bottom bag and connected the pooling bag with a 5 days storage bag, mixed well and low-speed centrifugation. Transferring the supernatant (LPPC) to a 5 days storage bag by manual separator, then measured the volume and calculated the volume of PAS for adding in LPPC. Connected the product with PAS by sterile connecting device and added PAS in the ratio of plasma 35% and PAS 65% in accordance the standard reference.
The Second Trial
PCs were pooling four iso-group buffy coats with PAS only. We connected them with sterile connecting device and washed buffy coat with 250 ml of PAS from the top bag to the bottom bag. The pooling bag was connected with a 5 days storage bag, mixed well and low-speed centrifugation. The supernatant (LPPC in PAS) transferred to a 5 days storage bag by manual separator.
Results
The First Trial
The total numbers of LPPC in plasma were 175 units and LPPC in PAS were 30 units. LPPC in PAS had mean of volumes 304 ml, content of platelet concentrates 2.8 X1011 cells/unit compared with random platelet concentrates equal 5 units and had 0.1X109 white blood cells contamination. LPPC from traditional methods had mean of volumes 324 ml, contents of platelet concentrates 3.9 X1011 cells/unit compared with random platelet concentrates equal 7 units and had 0.1X109 white blood cells contamination. The platelet yields showed significant different (p>0.1), volume and white blood cells contamination were not significant different (p<0.1).
The Second Trial
The total numbers of LPPC in PAS were 45 units, had mean of
Procedure of preparing LPPC

volumes 300 ml, content of platelet concentrates 3.6 X1011 cells/ unit compared with random platelet concentrates equal 6.5 units and had 0.2X109 white blood cells contamination (Table 1).
The platelet yields, volume and white blood cells contamination were not significant different (p<0.1).
The Titer Detection
The titer of anti-A and anti-B in LPPC in PAS is less than or equal to 64, all of which are classified as low titer, but LPPC from the traditional way with a titer of anti-A and anti-B over 64 about 20% (Table 2).
Discussion
Statistical tests showed that there was no difference in volumes and white blood cells contamination from the two methods (P<0.1), but the content of platelet concentrates obtained from traditional methods over to new methods of statistical significance (P>0.1) in the first trial. However, the contents of platelet concentrates from LPPC in PAS provides reached the recommended quality of Council of Europe (EU) and National Blood Centre, Thai Red Cross Society (TRC) which equal or more than 2.4X1011 cells/unit In the second trial, there was no difference in volumes, white blood cells contamination and the content of platelet concentrates by the two methods (P<0.1). We used LPPC in PAS from the second trial method in our routine work. The reason of successful in the second trial is the large volume of PAS, we used 250 ml to wash buffy coat compared to the first trial, used only 100 ml of plasma to wash buffy coat from the system. The more volume of PAS or plasma could separate platelet concentrates better than small volume.
Transfusion of platelets in often resorted to, in the oncology world, when thrombocytopenia resulting from chemotherapy, radiation or bone marrow tumor infiltration leads to potential or active bleeding. It is also used to raise the platelet count in anticipation of invasive procedures or when anticoagulation is mandatory. Red blood cell transfusion is based on relatively rigorous criteria, such as the hemoglobin level, whereas platelet transfusion is often biased by the perceived risks of bleeding. Platelet transfusion is often indicated in oncology patients receiving myelo-suppressive chemotherapy resulting in significant thrombocytopenia with or without bleeding. It is also often used in patients receiving radiation and when heavy bone marrow infiltration by metastasis results in impaired platelet production [1].
In a nation survey conducted in the USA in 2011, 2,516,000 apheresis platelet units and 130,000 pooled units from whole blood were delivered to meet the needs for platelet transfusion [9]. A recent nationwide survey conducted in France revealed that hematologic and cancer pathologies included 46% of transfused patients, 34% of the patients had transfusions in a surgical context, and 32.4% of transfused patients were receiving medication with an impact on transfusion [10].
The use of ABO-mismatched platelets in oncology patients lead to decreased therapeutic benefit or adverse reactions such as refractoriness, mediated by ABO antibodies clearing platelets from the circulation minutes to hours after infusion [11]. The ABO incompatibility also promotes the HLA allo-immunization in the multi-transfused patients and has potential for a hemolytic transfusion reaction. Accordingly, ABO-matched platelets are recommended for oncology patients with low platelet counts where the use of apheresis products, having lower red cell contamination is preferred option. Despite the low numbers of residual red cells in routine platelet preparations collected from random Rh-positive donors, the risk of immunogenicity of the D-antigen is still present.
Allo-immunization can, therefore, be prevented with a dose of 50-300 ug of Rh immunoglobulin within 72 hours of D antigen exposure [12,13].
Platelet transfusion remains crucial to stop bleeding in thrombocytopenic cancer patients during their surgeries, chemotherapy and/or radiotherapy. The current implementation of newer validated suspension media and plasma reduction leads to platelets increasing shelf life and reduced allergic reactions. All of LPPC in PAS are classified as low titer, which led to the patient at any group, reduced red cell hemolysis from ABO incompatible plasma and reduce other adverse effects related to plasma transfusion [5-7].
References
- Thierry Burnouf, Mohamed Elemary, Julia Radosevic, et Platelet transfusion in thrombocytopenic cancer patients: Sometimes justified but likely insidious. Transfusion and Apheresis Science. 2017; 56: 305-309.
- Rock G, Swenson SD, Adams GA. Platelet storage in a plasma- free Transfusion. 1985; 25: 551-556.
- Holme S, Heaton WA, Courtright M. Improved in vivo and in vitro viability of platelet concentrates stored for seven days in a platelet additive Br J Haematol. 1987; 66: 233-238.
- Van der Meer PF, Pietersz RN, Tiekstra MJ, et WBC-reduced platelet concentrates from pooled buffy coats in additive solution: an evaluation of in vitro and in vivo measures. Transfusion. 2001; 41: 917-922.
- Vamvakas EC, Blajchman Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009; 113: 3406-3417.
- Heddle NM, Klama L, Singer J, et al. The role of the plasma from platelet concentrates in transfusion N Engl J Med. 1994; 331: 625-628.
- AABB Association Bulletin #10-06-Information Concerning Platelet Additive 2010.
- Kerkhoffs JL, van Putten WL, Novotny VM, et Dutch- Belgian HOVON cooperative group. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. Br J Haem. 2010; 150: 209-217.
- https://www.hhs.gov/sites/default/files/ash/bloodsafety/2011- pdf
- Fillet AM, Desmarets M, Assari S, et al. Blood products use in France: a nationwide cross-sectional survey. Transfusion. 2016; 56: 3033-3041.
- Chubak J, Whitlock EP, Williams SB, et Whitlock. Aspirin Use for the Prevention of Colorectal Cancer: An Updated Systematic Evidence Review for the US Preventive Services Task Force. 2015; 164: 814-825.
- Schulz WL, Snyder EL. Transfusion support for the oncology Rossi’s principle of transfusion medicine (5th ed), John Wiley & Son Ltd. 2016.
- Cooling ABO and platelet transfusion therapy. Immunohematology. 2007; 27: 20-33.